Comparison of Antifungal Properties of Gold, Silver, and Selenium Nanoparticles Against Amphotericin B-Resistant Candida glabrata Clinical Isolates
-
Lotfali, Ensieh
-
Department of Medical Parasitology and Mycology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Toreyhi, Hossein
-
Student Research Committee, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Makhdoomi Sharabiani, Kamyab
-
Student Research Committee, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
 Fattahi, Azam
Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 88961363; E-mail: afattahi@sina.tums.ac.ir
Fattahi, Azam
Center for Research and Training in Skin Diseases and Leprosy, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 88961363; E-mail: afattahi@sina.tums.ac.ir
-
Soheili, Amirali
-
Student Research Committee, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Ghasemi, Reza
-
Student Research Committee, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Keymaram, Mahyar
-
Department of Medical Parasitology and Mycology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
Rezaee, Yasaman
-
Student Research Committee, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Iranpanah, Sayna
-
Student Research Committee, Faculty of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Abstract: Background: The present study aimed to investigate the antifungal activity of Nanoparticles (NPs) against amphotericin B-resistant Candida glabrata (C. glabrata) strains.
Methods: Twelve resistant (C. glabrata) strains were isolated from archived clinical isolates. Antifungal activity was conducted according to Clinical and Laboratory Standards Institute’s (CLSI) guidelines, document M27-A3/S4. The Scanning Electron Microscope (SEM) was used to observe the morphological changes of strains exposed to each nanoparticle.
Results: Minimum Inhibitory Concentration (MIC) of nanoparticles of all strains was in the concentration range of 0.125 to 0.5 µg/Ml. The synthesized Ag-NPs showed superior antifungal activity against (C. glabrata) compared to Se-NPs and Au-NPs. The scanning electron microscope images revealed the difference in the fungal morphology between the untreated and treated fungi with nanoparticles.
Conclusion: The Ag-NPs, followed by Se-NPs synthesized, revealed significant antifungal activity against resistance regardless of their antifungal-resistant mechanisms.
Introduction :
Candida glabrata (C. glabrata) is one of the most common opportunistic human fungal pathogens, which is responsible for 90-100% of mucosal infections and invasive candidiasis with the high mortality rate in immunocompromised and critically ill individuals 1. Over the past decade, the improper and misuse of existing antifungal drugs have led to the development of drug-resistant clinical isolates of Candida species (spp.). The classes of antifungal drugs active against C. glabrata include pyrimidine analogs (Flucytosine), azoles, echinocandins, and polyenes 2. Resistance to amphotericin B is rare, however, a change in the amount of ergosterol or production of different types of sterol that construct fungal cell membranes is the probable mechanism for this resistance without causing a major challenge in normal cell functionality 2.
The synthesis of Nanoparticles (NPs) has been studied for some time, and various attempts have been made to apply them to advance the field of nanomedicine. Nanoparticles have diverse features depending on the metallic element, size, and shape of the particles. Specifically, silver nanoparticles are well-known for their biocidal properties, including antibacterial, antifungal, antiviral, and anticancer activities 3. The resistance of C. glabrata to routine antifungal therapy poses a major challenge to the management of infection, hence the use of other agents with antifungal property helps to improve the management of infection. Here, it is hypothesized that silver (Ag), selenium (Se) and gold (Au) nanoparticles could change cell wall structure in the resistant strains through forming pores, possibly improving the sensitivity of resistant isolates. The present study aimed to investigate the antifungal activity of Ag-NPs, Se-NPs, and Au-NPs against amphotericin B-resistant C. glabrata strains.
Materials and Methods :
Fungal strain: Twelve resistant C. glabrata strains (Table 1) were isolated from the vaginal tract of patients in an epidemiological study of antifungal resistance. This study was performed at Department of Medical Parasitology and Mycology of Shahid Beheshti University of Medical Sciences. C. glabrata (ATCC 90030) was used as a control strain. Antifungal activity of three NPs, including Ag-NPs, Se-NPs, and Au-NPs were investigated according to Clinical and Laboratory Standards Institute’s (CLSI) guidelines mentioned in the document M27-A3/S4 4.
Synthesis and characterization of NPs: The nanoparticles (Sigma, USA) were prepared according to the previous methods 5. For the characterization of size and charge of NPs, the Z-average size and zeta potential were estimated using the Malvern Zetasizer Nano ZS-90 instrument (Malvern, England). A Scanning Electron Microscope (SEM) (AIS2100, Seron Technology, South Korea) was used to observe the morphological changes of C. glabrata strains exposed to the NPs (Ag, Se, Au) 6.
Statistical analysis: All the data of the antifungal activity were expressed as mean±SD. The significant differences were defined at p<0.05.
Results :
Characteristics of NPs: The zeta-average sizes of Au, Se, and Ag-NPs were ranged from 10-15, 105-209, and 30 nm, respectively. The zeta potentials of Au, Se, and Ag NPs were -22.4, -49.93, and -23.5 Mv, respectively.
Minimum inhibitory concentration of the NPs: After 24 hr of incubation, the Minimum Inhibitory Concentration (MIC) of nanoparticles of all C. glabrata strains was in the concentration range of 0.125 to 0.5 µg/ml (Table 1). No inhibition was observed in the low concentration range of ≤0.125 µg/ml; however, the growth was significantly inhibited at 0.125 µg/ml or more compared to the control. The synthesized Ag-NPs had superior antifungal activity against C. glabrata compared to Se-NPs and Au-NPs.
Scanning electron microscope image: In C. glabrata, the untreated C. glabrata (Figure 1A) was only spherical or oval-shaped blastoconidia, which were considerably smaller than another Candida spp. Once C. glabrata was treated with Ag-NPs (Figure 1B), abnormal morphology, some pores, and distorted membranes appeared in the yeasts (white arrows on the image). As shown in figure 1C, Se-NPs were attached and agglomerated to the surface of nearby yeasts (white arrows on the image). After treatment with NPs at the MIC dose, the cell membrane lost its smoothness and resulted in unusual surface bulges, inducing impairment of cell membrane integrity. At 2×MIC dose concentration of Ag-NPs and SeNPs (Figures 1B and 1C), the fungal morphology underwent a dramatic change from cylindrical to distorted cell structure with the breakdown of the cell membranes. However, at 2×MIC level concentration of Au-NPs (Figure 1D), the fungal morphology did not change much.
Discussion :
Regarding the present observation, NPs have excellent antifungal effects, because of their low MIC ranges and, consequently, have more potency against amphotericin B-resistant C. glabrata strains.
The results of antifungal activity against resistant C. glabrata strains after exposure to Ag-NPs with inhibitory action at a 0.125-0.5 µg/ml concentration were better as compared to Se-NPs and Au-NPs. The superior activity of Ag-NPs to inhibit the growth of various pathogenic fungi, such as Trichophyton and Microsporum spp, was reported 7. Ag-NPs followed by Se-NPs have not contributed to fungal resistance complicating antifungal therapy of candidiasis. Present results show that synthesized Ag-NPs have highly antifungal activity against resistant C. glabrata strains, which might be through the destruction of membrane integrity, resulting in the formation of pores and cell death (Figures 1B and 2B). It has been suggested that upon the treatment, the replication ability of DNA, expression of ribosomal subunit protein and the enzymes essential for ATP production are lost, leading to the inactivation of microorganisms 8.
The mechanism of inhibitory effect of NPs on resistant isolates of C. glabrata creates a scenario in which Ag-NPs/Se-NPs like different routine antifungals have a different fungistatic or fungicidal mechanism, including interference with cell wall synthesis and inhibition of ergosterol synthesis. It means that these agents do exert their fungistatic/fungicidal effects in a broad range of targets in the organisms. Based on the MIC results, it is safe to say that nanoparticles have an intensive antifungal property, making them a suitable alternative with regard to drug-resistant isolates.
Some studies have reported that the positive charge on the Ag+ ion is crucial for its antimicrobial activity through the electrostatic attractions between the negatively charged cell membrane of microorganisms and the positively charged nanoparticles 9.
In the present study, no antifungal activity of Au-NPs was observed in all tested isolates. Au-NPs exhibited antifungal activity against C. glabrata, Candida albicans (C. albicans), Candida krusei (C. krusei), Candida parapsilosis (C. parapsilosis), and Cryptococcus neoformans, while Candida tropicalis C. tropicalis and dermatophyte spp. revealed resistance to Au-NPs (MIC for dermatophyte spp.: 10-30 µg/ml) 10.
To sum up, the Ag-NPs, followed by Se-NPs synthesized, revealed significant antifungal activity against amphotericin B-resistant C. glabrata strains, disregarding their antifungal-resistant mechanisms. The activity analysis of nanoparticles indicated that the nanoparticles were capable of changing membrane and cell wall structure in the resistant strains, possibly through pore formation on the membrane and cell wall.
Conclusion :
This research revealed the fungicidal capacities of Ag-NPs followed by Se-NPs against amphotericin B-resistant C. glabrata strains. This information can be useful in encapsulating antifungal drugs in combination with the mentioned NPs to decrease the side effects and enhance the effectiveness of the drugs.
Conflict of Interest :
None declared.
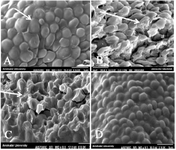
Figure 1. SEM images of C. glabrata treated with silver nanoparticles; 9000×magnification
- A) Untreated glabrata; the white arrow indicates the smooth cell membrane of normal yeast.
- B) glabrata treated with 0.5 µg/ml Ag-NPs.
- C) glabrata treated with 0.5 µg/ml Se-NPs.
- D) glabrata treated with 0.5 µg/ml Au-NPs.
|
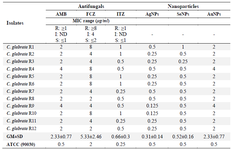
Table 1. Comparison MIC of 12 AMB-resistant C. glabrata strains exposure to Ag-NPs, Se-NPs, Au-NPs
Fluconazole (FCZ); Amphotericin B (AMB); Itraconazole (ITZ). S; Susceptible, I; Intermediate, R; Resistant, MIC: Minimum inhibitory concentration. No. of resistant C. glabrata strains (R1-R12). Geometric range; GM, Standard Deviation; SD.
|
|