Effects of Poly-N-isopropylacrylamide Microgels Containing Antibiofilm Substances on Pseudomonas aeruginosa Isolated from Chronic Wounds
-
Etemadinia , Akram
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Science, Tehran, Iran
-
Seyfoori , Amir
-
Department of Biomaterials and Tissue Engineering, Breast Cancer Research Center, Motamed Cancer Institute, ACECR, Tehran, Iran
-
Rahimi Foroushani , Abbas
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Science, Tehran, Iran
-
Mazaheri Nezhad Fard, Ramin
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Science, Tehran, Iran
-
Food Microbiology Research Center, Tehran University of Medical Science, Tehran, Iran
-
 Bakhtiari , Ronak
Department of Pathobiology, School of Public Health, Tehran University of Medical Science, Tehran, Iran, Tel:+98 21 42933051; E-mail: rounakbakhtiari@yahoo.com
Bakhtiari , Ronak
Department of Pathobiology, School of Public Health, Tehran University of Medical Science, Tehran, Iran, Tel:+98 21 42933051; E-mail: rounakbakhtiari@yahoo.com
Abstract: Background: Biofilm formation helps Pseudomonas aeruginosa (P. aeruginosa) survive in various environments. Microgels can be effective in treatment of bacterial infections. The major aim of this study was to investigate effects of poly-N-isopropyl-acrylamide microgels (PNIPAM) on P. aeruginosa.
Methods: Totally, 100 P. aeruginosa strains were isolated from chronic wound infections. Quantitative assessments of biofilm formation and antibiotic susceptibility were carried out. Furthermore, algD, lasR, and PA2714 genes were amplified to investigate gene frequencies and expression rates.
Results: Significant decreases were seen in lasR expression in EDTA-treated samples. Significant decreases were observed in expression of algD and lasR treated with xylitol. Decreased expression of PA2714 was seen in samples treated with xylitol with no significance.
Conclusion: The PNIPAM containing xylitol or EDTA could penetrate biofilms of P. aeruginosa and significantly decrease expression of lasR and algD. This can be a novel strategy in the management of chronic wounds.
Introduction :
Pseudomonas aeruginosa (P. aeruginosa) is a Gram negative, opportunistic nosocomial pathogen with biofilm formation characteristics 1. The formation of biofilm in wounds is one of the most important complications in wound healing. Biofilms are resistant to antibiotics, increasing treatment costs and failures. Poly-N-isopropylacrylamide microgel (PNIPAM) is one of the most popular polymers for the replacement of antibiotics in biofilm formation inhibition. The major aim of this study was to assess effects of PNIPAM containing antibiofilm substances on P. aeruginosa isolated from chronic wound infections as well as investigating antibiotic susceptibility patterns and prevalence of algD, lasR, and PA2714 genes of these isolates. Moreover, effects of microgels containing antibiofilm substances on expression of the highlighted genes were assessed.
Materials and Methods :
Sample collection: In total, 100 P. aeruginosa were isolated from patients with chronic wound infections in four affiliated hospitals of Tehran University of Medical Science, Tehran, Iran. Bacteria were identified using phenotypic assays as well as molecular assays using specific primers for rpsL genes producing 126-bp amplicons and Polymerase Chain Reaction (PCR) (Bio-Rad, USA) 2.
Antibiotic susceptibility assessment: Antibiotic susceptibility assessment of the bacteria against imipenem (10 mg), ceftazidime (30 mg), gentamicin (10 mg), piperacillin-tazobactam (100 mg), ciprofloxacin (5 mg), ticarcillin (75 mg), tobramycin (10 μg), piperacillin (100 mg) and cefepime (30 mg) was carried out using disk diffusion method.
Quantitative assessment of biofilm formation: A microtiter plate (MTP) assay was used for the quantitative detection of biofilms. Generally, test OD was compared with control OD as OD>0.33296 show-ed strong biofilm formation, 0.33296>OD>0.16648 showed moderate biofilm formation, 0.16648>OD>0.08324 demonstrated weak biofilm formation and OD<0.8324 showed no biofilm formation. The P. aeruginosa ATCC 27853 was used as positive control.
Verification of biofilm formation using SEM: Verification of biofilm formation by the bacteria was carried out using Scanning Electron Microscopy (SEM). Briefly, the surfaces of the bacterial isolates were micro-coated with gold and studied at 5 and 10-μm magnifications using SEM (Shimadzu SSX-500, Shimadzu, Japan).
PNIPAM synthesis: To synthesize PNIPAM, a two-step precipitation polymerization reaction was used to achieve homogeneous cross-linked microgels. Briefly, colloidal microgels were prepared and purified via dialysis against deionized water for 1 w. Purified microgel solutions were concentrated to produce dried microgel foams using a freeze-dryer (Christ Alpha 1-4, Palaiseau Cedex, France) 3.
Assessment of PNIPAM using zeta-sizer: Assessment of PNIPAM was carried out using the Dynamic Light Scattering (DLS) technique (Malvern Zeta-sizer, Malvern Instrument, UK) 4.
PNIPAM loading with xylitol and EDTA: Briefly, PNIPAM dried microgel foams were mixed with xylitol or Ethylenediaminetetraacetic acid (EDTA) solution (0.1 N) at 4°C for 24 hr, then centrifuged at 12000 g and washed three times with Phosphate-Buffered Saline (PBS).
Investigation of xylitol and EDTA releases from the loaded microgels: Drug release investigation was carried out by preparing 1 mg ml-1 suspensions of xylitol or EDTA-loaded PNIPAM in 2 ml of PBS at 37°C. Microgels were precipitated at various time intervals using a centrifuge at 12000 g. At each time interval, 500 µm of the supernatant was collected. Concentrations of xylitol and EDTA in supernatants were quantified at 230 nm using UV-visible spectroscopy (JASCO, Japan) 5.
Treatment of the bacterial isolates with PNIPAM containing xylitol and EDTA: Isolates were cultured in nutrient broth and incubated at 37°C for 24 hr to reach 0.5 McFarland turbidity. For each milliliter of nutrient broth, 10 µl of the microgel solution loaded with xylitol or EDTA were added to the bacterial tubes, incubated at 37°C for 4 hr and centrifuged at 12000 g for 3 min 3. Then, supernatants were discarded and the bacterial precipitates were stored at -20°C until RNA extraction.
FT-IR analysis of microgels loaded with xylitol and EDTA: Fourier Transform-Infrared spectroscopy (FT-IR) (Spectrum Two, USA) was used to analyze microgels loaded with xylitol and EDTA 6. The wavelength range was set at 4000-500 cm-1 at a resolution of 4 cm-1. Absorbances of the peaks were compared with those of the reference peaks at A1655/A3450.
Gene detection and expression assessment using conventional and real-time PCR techniques: Treated and non-treated (control) isolates were cultured on Mueller-Hinton agar at 37°C for 24 hr. Then, DNA and RNA were extracted using Favorgen (Taiwan) and Wizibio (South Korea) kits, respectively. Bacterial DNA was used in PCR of algD, lasR and PA2714. Furthermore, cDNA was synthesized from the bacterial mRNA using random primers and reverse transcriptase enzyme (Thermo Scientific, USA) and then quantified using specific primers and a real-time PCR machine (Bioneer, South Korea). The rpsL gene was amplified as an internal control.
Statistical analysis: Statistical analysis was carried out using SPSS Software v.24 (IBM, USA) and chi-square and Fisher’s tests. Statistical analysis of the real-time PCR data was carried out using REST Software. Significant levels were recorded at p<0.05.
Results :
In total, 100 bacterial strains were isolated from chronic ulcer samples, including diabetic, intravenous and bed and trauma-induced ulcers as well as burn wounds. Diabetic foot included the highest number of patients and intravenous wounds involved the oldest patients. The highest antibiotic resistance rates belonged to tocicarlsin (83%), tobramycin (81%) and ceftazidime (80%), respectively (Figure 1). Of the total P. aeruginosa isolates, 59 isolates (59%) formed strong biofilms, 21 isolates (21%) formed moderate biofilms, 18 isolates (18%) created weak biofilms and two isolates (2%) formed no biofilms. Bacterial biofilm formation was successfully verified using SEM (Figure 2). Results of the average and distribution size assessments of PNIPAM are shown in figure 3. Figure 4 shows cumulative release profiles of the microgels within 48 hr of the extraction. In general, rapid releases of EDTA from the microgels were seen as nearly 40% of the antibiofilm compounds were released from the microgels within 6 hr. However, the second step was slow and the rest of the compound was released within 48 hr; where ʎmax was 250 nm and the proportion of the loading was 68.25% ±1.57.
The xylitol release profile was two-stepped as well. In general, the xylitol release rate was higher than that of EDTA with nearly 48% of antibiofilm released within the first 6 hr. In the second stage, releasing process was relatively slower and the rest of the composition was released within 48 hr. Analysis results of the microgels loaded with xylitol and EDTA are demonstrated in figure 5. Frequencies of algD, lasR and PA2714 in the 59 isolates included 56 (94.9%), 53 (89.9%) and 48 (81.3%), respectively. Significant decreases in lasR expression were observed in EDTA-treated samples, compared with those in untreated ones (p=0.036). Expression changes of algD and PA2714 decreased in EDTA-treated samples while non-significant and significant decreases were respectively reported in expressions of algD and lasR treated with xylitol (p=0.036). Expression of PA2714 decreased in xylitol-treated samples with no significance (p=0.053).
Discussion :
In total, 100 bacterial strains were isolated from chronic ulcer samples. Diabetic foot included the highest number of patients. Rahim K et al reported 7 that diabetic wounds were the highest prevalent (43.96%) wounds infected by P. aeruginosa, which was higher than that reported by the present study (32%). Diabetic foot infection is the most common and severe complication in patients with diabetes. In the highlighted study, 97.5% of P. aeruginosa isolates produced biofilms. Similarly, 98% of P. aeruginosa isolates produced biofilms in the present study. Biofilm formation of bacteria in chronic wounds is the major reason for the frequent treatment failures of antibiotics and disinfectants 8. In a study by Rofooei A et al on P. aeruginosa isolates, 55% of the isolates produced strong biofilms, while 45% of the isolates formed weak biofilms 9. These results were similar to the results from the present study.
In the present study, the highest antibiotic resistance belonged to tocicarlsin (83%), tobramycin (81%) and ceftazidime (80%). Similarly, Rofooei A et al reported that P. aeruginosa was highly resistant to ceftriaxone, ceftazidime, cefotaxime (70%), piperacillin (35.08%) and amikacin (39.59%), respectively 9. The major reason for this resistance included the extensive use of antibiotics. However, rates of antibiotic resistance in the present study were higher than those in the highlighted study. In the present study, 48 (81.3%) isolates included the PA2714 gene, indicating the high presence of this gene and its importance in biofilm formation. The present study indicated that PNIPAM loaded with EDTA or xylitol significantly decreased the expression of the genes involved in the formation of biofilms. In a study by Ammons et al, the effects of a hydrogel formulated with lactoferrin and xylitol (as antibiofilm compounds) in combination with silver were investigated. Results showed that these compounds inhibited biofilms 10.
Conclusion :
Results showed that PNIPAM loaded with xylitol or EDTA could penetrate biofilms of P. aeruginosa. This significantly decreased expression of lasR and algD. In conclusion, use of PNIPAM loaded with xylitol or EDTA can be addressed as a novel strategy in the management of chronic wound healing.
Acknowledgement :
The authors thank the staff of the Microbiology Laboratory. This study was financially supported by a grant from the Deputy of Research, Tehran University of Medical Sciences (Grant No. 98.01.27.41428).
Conflict of Interest :
The authors declare no conflict of interest.
Ethical approval :
This study was approved by the Ethical Committee of Tehran University of Medical Sciences (Ethical Code: IR.TUMS.SPH.REC.1397.316).
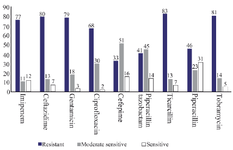
Figure 1. Antibiotic susceptibility schemes of Pseudomonas aeruginosa isolates.
|

Figure 2. Verification of the biofilm formation by Pseudomonas aeruginosa isolates using SEM.
|
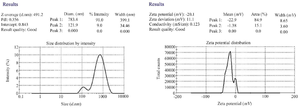
Figure 3. Results of the physical assessment of PNIPAM synthesis using zeta-sizer.
|
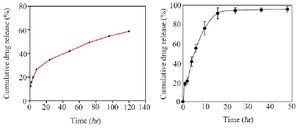
Figure 4. Release profiles of EDTA (right) and xylitol (left) from the loaded microgels at various time intervals.
|

Figure 5. Results from the FT-IR of PNIPAM synthesis.
|
|