Antiplasmodial Activity of Green-Synthesized MgO Nanoparticles Using Achillea millefolium Against Chloroquine-Resistant and-Sensitive Plasmodium falciparum
-
Khamsehpour, Niloufar
-
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Hanifian, Haleh
-
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
 Nateghpour, Mehdi
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 9123278020; E-mail: nateghpourm@sina.tums.ac.ir
Nateghpour, Mehdi
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 9123278020; E-mail: nateghpourm@sina.tums.ac.ir
-
Research Center for Quran, Hadith and Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
 Raeisi, Ahmad
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 9123278020; E-mail: raeisia@tums.ac.ir
Raeisi, Ahmad
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran , Tel: +98 9123278020; E-mail: raeisia@tums.ac.ir
-
Shabani, Mohammad
-
Department of Biochemistry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
Khezri, Aram
-
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Dehdast, Seyed Ahmad
-
Center for Research Endemic Parasites of Iran, Tehran University of Medical Sciences, Tehran, Iran
-
Farivar, Leila
-
Department of Medical Parasitology and Mycology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Shahsavari, Saeed
-
Department of Biostatistics and Epidemiology, School of Health, Alborz University of Medical Sciences, Karaj, Iran
Abstract: Background: Resistance to antimalarial medications, particularly in Plasmodium falciparum (P. falciparum), has emerged as a significant challenge, highlighting the need for innovative therapeutic strategies. Green-synthesized magnesium oxide nanoparticles (MgO NPs) represent a promising approach to therapeutic interventions. This study presents one of the first detailed evaluations of green-synthesized MgO NPs derived from Achillea millefolium (A. millefolium) against both chloroquine-sensitive (3D7) and chloroquine-resistant (K1) P. falciparum strains.
Methods: In this study, MgO NPs were biosynthesized using A. millefolium extracts with varying solvent ratios. The nanoparticles were characterized using UV-Vis, FTIR, FESEM, and DLS techniques. Cytotoxicity was assessed via MTT and hemolysis assays. Their antiplasmodial efficacy was evaluated in vitro against chloroquine-sensitive (3D7) and -resistant (K1) P. falciparum strains.
Results: The synthesized MgO NPs displayed quasi-spherical morphology and nanoscale size. Among tested formulations, the most effective showed IC₅₀ values of 0.17 mg/ml for the 3D7 strain and 0.76 mg/ml for the K1 strain, indicating significant antiplasmodial activity.
Conclusion: Green-synthesized MgO NPs using A. millefolium demonstrated potent antiplasmodial activity at low IC₅₀ concentrations, showing efficacy against both chloroquine-sensitive and -resistant P. falciparum strains. These findings highlight their promise as plant-based nanotherapeutics for malaria treatment.
Introduction :
Malaria remains one of the leading causes of mortality worldwide; however, prompt detection and timely intervention can prevent severe outcomes 1. The treatment of malaria is becoming increasingly challenging due to the emergence of drug-resistant Plasmodium falciparum (P. falciparum) strains, which exhibit resistance to nearly all currently available antimalarial agents. This poses a critical obstacle to effective disease management. Although chemotherapy has historically played a pivotal role in malaria control, its efficacy is now undermined by rising treatment failures, underscoring the urgent need for novel therapeutic strategies 2. Medicinal plants continue to serve as a valuable source of bioactive compounds with antimalarial potential, offering benefits such as reduced toxicity, cost-effectiveness, and physiological compatibility. However, their clinical application is often limited by poor solubility, low bioavailability, and in vivo instability.
Recent advances in nanotechnology have opened promising avenues for overcoming these limitations by enabling the development of nano-based drug delivery systems that enhance the pharmacological properties of plant-derived compounds 3. Achillea millefolium L., a medicinal herb rich in flavonoids, phenolic acids, and terpenes, has demonstrated antimicrobial, antioxidant, and anti-inflammatory activities 4. Although it has shown potential in managing parasitic and infectious diseases, its application against malaria has not yet been thoroughly investigated 5,6.
In the present study, a novel green synthesis approach was introduced for the fabrication of magnesium oxide nanoparticles (MgO NPs) using aqueous and ethanol extracts of Achillea millefolium (A. millefolium). To the best of our knowledge, this is the first report utilizing A. millefolium for MgO NP synthesis with an emphasis on antiplasmodial evaluation. This phytochemical-assisted method offers several advantages: it is conducted at 80°C temperature and pressure, does not require chemical catalysts or thermal annealing, and utilizes the plant extract as a natural chelating and reducing agent, ensuring an eco-friendly and energy-efficient process.
Previous studies have highlighted the antimicrobial and antiparasitic properties of MgO NPs synthesized through various green and chemical routes, particularly against bacterial and fungal pathogens. However, their antiparasitic potential, especially against Plasmodium spp., remains underexplored. This study addresses this gap by evaluating the biological activity of synthesized MgO NPs using A. millefolium, with a specific focus on their antimalarial effects.
Materials and Methods :
Plant extraction: The extraction of A. millefolium was conducted at Iran University of Medical Sciences. Dried flowers were separated from roots, ground into fine powder, and 1 g of the powder was mixed with 100 ml of solvent in varying ratios of Distilled Water (DW) and 96% ethanol (EtOH): 50:50, 70:30, and 30:70 (DW: EtOH). Each mixture was stirred at 200 rpm using a magnetic stirrer and subjected to reflux extraction at 70°C for 3 hr. The mixtures were then cooled to room temperature and filtered using Whatman No. 1 filter paper.
Biosynthesis of MgO NPs: Green synthesis of MgO NPs was carried out using 5 ml of A. millefolium extract (prepared in the above DW: EtOH ratios) mixed with 15 ml of distilled water and heated to 60°C for 5 min. Then, 0.1 g of Magnesium chloride (MgCl₂) was added as the metal precursor and the mixture was stirred at 300 rpm. The reaction was maintained at 80°C for 3 hr, based on the method described by Verma et al 7. The bioactive phytochemicals in the extract served as reducing and stabilizing agents, promoting the reduction of Mg²⁺ ions and formation of MgO NPs. The resulting suspensions were collected for physicochemical characterization and biological evaluation.
Characterization: MgO NPs were characterized using UV-visible spectroscopy (UV-2450, PerkinElmer), Fourier-transform infrared spectroscopy (FTIR; Tensor 27, Bruker, Germany), field emission scanning electron microscopy (FESEM; TESCAN MIRA3, Czech Republic), dynamic light scattering (DLS; HORIBA SZ-100), and zeta potential analysis (HORIBA SZ-100). The UV-visible spectrum was recorded from 200-800 nm. FTIR analysis was used to identify functional groups involved in nanoparticle synthesis. FESEM assessed surface morphology, while DLS and zeta potential provided information on size distribution and colloidal stability, respectively.
Hemolysis assay: Hemolytic activity of the A. millefolium extracts and MgO NPs was evaluated on human erythrocytes using a spectrophotometric method. Fresh heparinized O⁺ blood was washed thrice with Phosphate-Buffered Saline (PBS, ph=7.0) and suspended in normal saline to a concentration of 1%. A stock solution (total volume 1000 µl) was prepared by combining 660 µg/ml of extract or MgO NPs with 340 µg/ml of normal saline. Serial dilutions (0.125, 0.25, 0.5, and 1 mg/ml) were prepared and mixed with erythrocyte suspensions. After 1 hr of incubation at 37°C, samples were centrifuged and the absorbance of the supernatant measured at 541 nm. Hemolysis percentage was calculated as:
% hemolysis=HbABSHbHb100 % ABS ×100
Cultivation of P. falciparum and preparing complete culture medium (CCM): P. falciparum strains 3D7 and K1 were cultured using the Trager and Jensen method with modifications. The Complete Culture Medium (CCM) consisted of RPMI-1640 supplemented with HEPES, 50 mg/L hypoxanthine, 50 mg/L gentamicin, and Human AB+serum. Infected blood samples (two drops) were added to 4.5 ml CCM. Non-infected erythrocytes were prepared by washing whole blood three times with saline, centrifuged at 5000 rpm for 5 min, and used to adjust hematocrit to 10%. Cultures were maintained at 37°C in a humidified incubator with 5% CO₂, 5% O₂, and 90% N₂. Subculturing occurred every 48 hr until parasitemia reached 2% (K1) or 3% (3D7).
MTT assay: Cytotoxicity of synthesized MgO NPs (from 30:70 and 50:50 DW: EtOH extracts) was assessed on Human Dermal Fibroblasts (HDF) via MTT assay. HDF cells were cultured in DMEM-High Glucose medium (Biowest, USA) supplemented with penicillin/ streptomycin (Gibco, USA) at 37°C with 5% CO₂. After trypsinization and neutralization, cells were seeded in 96-well plates. MTT reagent (Sigma-Aldrich, USA) was added and incubated for 3 hr. After removing the medium, 100 µl of DMSO (Bioldea, Iran) was added to dissolve formazan crystals. Absorbance was read at 570-630 nm (BioTek, USA). Cell viability was calculated as:
Cell survival rate = Optical absorption in the experimental groupOptical absorption in the control group ×100
In vitro antiplasmodial assay: Stock solutions of MgO NPs derived from A. millefolium extracts were prepared in RPMI medium at concentrations of 0.125, 0.25, 0.5, and 1 mg/ml. Chloroquine was used as a standard reference drug, formulated in the same medium at concentrations of 1, 5, 25, and 50 µg/ml. Additionally, MgCl2 was assessed in RPMI at equivalent concentrations of 0.125, 0.25, 0.5, and 1 mg/ml. Each stock solution was utilized to prepare serial dilutions of MgO NPs in a 96-well microplate, with positive control wells containing RPMI with infected Red Blood Cells (iRBCs) and negative control wells containing RPMI with uninfected RBCs. All assays were conducted in triplicate, with 20 µl of infected RBCs added to each well. Following a 24-hr incubation period at 37°C, parasitemia levels were assessed using Giemsa staining methodology. This staining technique is essential for visualizing the presence of parasites within the erythrocytes. The effectiveness of the various extracts was quantitatively evaluated by calculating the average percentage suppression of parasitemia, employing the following formula:
% Inhibition= Mean %parasitemia of untreated group-Mean% parasitemia of treated groupMean %parasitemia of untreated group×100
Statistical analysis: Data analysis was conducted using GraphPad Prism, employing two-way ANOVA for statistical testing. Significance levels were indicated as follows: * for p<0.05, ** for p<0.01, *** for p<0.001, and **** for p<0.0001.
Results :
Characterization of MgO Nps by UV-VIS Spectroscopy: To confirm the successful synthesis of MgO NPs, UV–Vis spectroscopy was performed to evaluate their optical properties. As shown in figure 1, absorbance measurements were recorded across the 200-800 nm range, which aligns with previously published studies on MgO NPs 8. The spectrum exhibited a prominent absorption peak between 250 nm and 300 nm, characteristic of MgO NPs synthesized via green methods using A. millefolium extract. This finding corroborates earlier studies and confirms nanoparticle formation.
Fourier transform infrared spectroscopy (FTIR): The FTIR analysis was performed to identify the functional groups of bioactive molecules in the A. millefolium extract involved in reduction, capping, and stabilization of MgO NPs. Figure 2 illustrates spectral differences between the plant extracts and the synthesized MgO NPs. The broad peak observed between 3200–3400 cm⁻¹ corresponds to O–H stretching vibrations of hydroxyl groups present in phenols and alcohols. The peak at 2979 cm⁻¹ is related to asymmetric and symmetric C–H stretching vibrations of methyl groups. A peak at 1642 cm⁻¹ indicates C=C stretching characteristic of alkenes, while bands at 1384 cm⁻¹ correspond to C–H bending or O–H bending in phenolic compounds. Peaks in the range of 1080–1043 cm⁻¹ indicate C–O stretching vibrations typical for alcohols and ethers. Importantly, the peak between 450–750 cm⁻¹ confirms metal–oxygen (Mg–O) bond formation, verifying the successful synthesis of MgO NPs 9.
Field Emission Scanning Electron microscopy (FE-SEM): The morphology and size of the sample were examined using Scanning Electron Microscopy, as illustrated in figure 3. Analysis of the obtained images reveals that the synthesized MgO NPs exhibit a size range of 20 nm to 60 nm (with a mean size of approximately 40 nm). Furthermore, the green-synthesized MgO NPs appear quasi-spherical and tend to form agglomerates, likely attributable to the interactions and van der Waals forces present among the particles 9,10.
Particle size and surface analysis of MgO NPs: Dynamic Light Scattering (DLS) analysis showed that the hydrodynamic diameters of MgO NPs varied depending on the extraction solvent ratio: 126.2 nm for 30:70 DW: EtOH, 161 nm for 50:50 DW: EtOH, and 356.9 nm for 70:30 DW: EtOH formulations (Figure 4). The presence of a single sharp peak in each case suggests a relatively homogeneous particle size distribution. Zeta potential measurements revealed low surface charges of −2.2 mV, +12.3 mV, and −2.3 mV for the three formulations, respectively (Figure 5). These relatively low zeta potential values indicate moderate colloidal stability, as values above ±30 mV are typically required for good electrostatic stabilization in suspension.
Hemolysis assay on human erythrocytes: The hemolytic activity of both A. millefolium extracts and MgO NPs was quantified (Figure 6). The results show that MgO NPs caused significantly less hemolysis compared to the crude plant extracts, suggesting better hemocompatibility of the synthesized nanoparticles.
MTT assay: The MTT assay was conducted to evaluate the cytotoxicity of MgO NPs on HDF cells. Concentrations tested ranged from 0.125 to 1 mg/ml. As shown in figure 7, a dose-dependent decrease in cell viability was observed, with MgO NPs synthesized using the 30:70 DW: EtOH formulation exhibiting lower cytotoxicity than the 50:50 formulation. Statistical significance was assessed via two-way ANOVA, and error bars represent the standard deviation of triplicate experiments.
Anti-plasmodial effects of synthesized MgO NPs: The antiplasmodial activity of MgO NPs was evaluated against chloroquine-sensitive (P. falciparum 3D7) and chloroquine-resistant (P. falciparum K1) strains. The IC₅₀ values against 3D7 were 0.25 mg/ml for 50:50 DW: EtOH, 0.38 mg/ml for 70:30 DW: EtOH, and 0.17 mg/ml for 30:70 DW: EtOH formulations. Against the resistant K1 strain, IC₅₀ values were higher: 0.76 mg/mL, 0.84 mg/ml, and 1.04 mg/ml, respectively. For comparison, chloroquine’s IC₅₀ values were 0.17 mg/ml (3D7) and 7.35 mg/ml (K1). The 30:70 DW: EtOH formulation demonstrated the most potent antiplasmodial activity, comparable to chloroquine against the sensitive strain (Table 1, Figures 8 and 9). This enhanced activity may relate to increased extraction of bioactive compounds with ethanol-rich solvent, contributing to better nanoparticle efficacy.
Discussion :
Malaria remains a significant global health challenge, especially in tropical and subtropical regions. This is largely driven by the increasing emergence of drug-resistant P. falciparum strains. Despite progress in vector control and antimalarial treatments, the limited availability of new therapeutic agents calls for alternative strategies. In this study, the successful green synthesis of MgO NPs using aqueous and ethanol extracts of A. millefolium, a medicinal plant known for its antimicrobial and antiparasitic properties was reported. This environmentally friendly and cost-effective synthesis approach is scalable and offers promising potential for sustainable nanomedicine development 11-16.
UV-Vis spectrophotometric analysis revealed a prominent absorption peak between 250 and 300 nm (Figure 1), consistent with previously reported biologically synthesized MgO NPs 17,18. This absorption confirms the formation of MgO NPs and their characteristic nanoscale optical properties. FTIR spectra (Figure 2) identified functional groups including hydroxyl, carbonyl, and ether bonds, suggesting that phenolic compounds and flavonoids in A. millefolium play key roles in the reduction and stabilization of Mg²⁺ ions during synthesis. These findings align with previous reports where phytochemicals from medicinal plants such as Ocimum sanctum and Azadirachta indica facilitated nanoparticle biosynthesis 19-21.
FESEM analysis provided direct visualization of the nanoparticle morphology, revealing quasi-spherical MgO NPs with diameters ranging from 20 to 60 nm (Figure 3). This morphology aligns with previous reports of spherical MgO NPs synthesized using plants such as Clitoria ternatea and Emblica officinalis, which exhibited particle sizes between 25 and 60 nm 14. Such nanoscale dimensions are known to enhance cellular penetration and interaction with biological targets due to their high surface area-to-volume ratios.
DLS analysis revealed a size distribution ranging from 126.2 to 356.9 nm, depending on the ethanol: water ratio used in extraction (Figure 4). The discrepancy between DLS and SEM data is expected due to hydrodynamic diameter inclusion and aggregation in solution, as previously reported by Gatou et al 22. Zeta potential analysis showed that only the 50:50 DW: EtOH formulation exhibited moderate colloidal stability (12.3 mV), while the other two had low surface charges (−2.2 and −2.3 mV) (Figure 5). These results indicate that nanoparticle formulations are sensitive to extraction solvent polarity, which may affect not only physical stability but also biological interactions.
Hemocompatibility testing revealed that MgO NPs induced significantly lower hemolysis compared to the crude A. millefolium extract (Figure 6), suggesting that nanoparticle formulation may reduce the toxicity of bioactive plant compounds when administered systemically. This observation is important when considering therapeutic applications in humans. In addition, MTT cytotoxicity assays conducted on HDF cells indicated a dose-dependent reduction in cell viability, with the (30:70) ethanol: water formulation being the least cytotoxic (Figure 7). The lower cytotoxicity may be associated with differences in phytochemical content and resulting particle characteristics such as size and surface charge. Similar findings were reported in previous studies, where green-synthesized MgO or Ag NPs showed minimal toxicity to normal human cells at effective concentrations 21. Selectivity Index (SI) calculations, based on IC₅₀ values for HDF cells vs. P. falciparum, revealed the highest selectivity for the (30:70) formulation (SI=3.6 for 3D7 strain; 0.59 for K1), indicating better parasite specificity, especially for the chloroquine-sensitive strain. The other formulations showed lower SI values, highlighting the need for further optimization to enhance safety and efficacy.
The core novelty of this study lies in its evaluation of the antiplasmodial activity of A. millefolium-derived MgO NPs against both chloroquine-sensitive (3D7) and chloroquine-resistant (K1) strains of Plasmodium falciparum. Among the three tested formulations, the (30:70) extract-based MgO NPs exhibited the lowest IC₅₀ (0.17 mg/ml) against 3D7, comparable to chloroquine itself (Figure 8, Table 1). Against the K1 strain, the same formulation showed an IC₅₀ of 1.04 mg/ml, which, although higher, still indicates meaningful antiplasmodial activity. The lower efficacy against K1 may stem from parasite-specific resistance mechanisms that affect cellular uptake or detoxification of nanoparticles.
Mechanistically, the observed antiparasitic effects may be attributed to several interrelated factors. First, the small particle size of MgO NPs enhances their internalization into infected erythrocytes, where they may interact directly with the parasite. Second, MgO NPs are known to generate Reactive Oxygen Species (ROS), which can disrupt intracellular redox homeostasis, damage parasite membranes, or interfere with DNA replication. Studies on ZnO and Ag NPs have demonstrated similar effects on protozoan parasites, including Leishmania and Plasmodium spp. 22-27. Third, the surface-bound phytochemicals from A. millefolium may synergistically contribute to antiplasmodial activity through membrane disruption or enzyme inhibition. The specific contribution of each mechanism requires further biochemical investigation.
The present study’s findings are consistent with previous studies on other green-synthesized metallic nanoparticles with antiparasitic properties. For example, Azadirachta indica-derived Ag NPs inhibited P. falciparum 3D7 and RKL9 strains with IC₅₀ values of 8–11 µg/ml 28, while Euphorbia hirta-mediated Ag NPs showed an IC₅₀ of 100 µg/ml 29. Although the IC₅₀ values in the present study are somewhat higher, the use of MgO—a metal oxide generally considered less toxic and more biocompatible than silver, may offer advantages in terms of safety, scalability, and regulatory approval.
Despite encouraging results, this study has limitations. It was restricted to in vitro models and did not assess pharmacokinetics, biodistribution, or detailed mechanistic pathways. Future work should involve in vivo validation, toxicity profiling, and mechanistic studies to unravel the molecular interactions between green-synthesized MgO NPs and Plasmodium parasites.
Conclusion :
In this study, a novel green synthesis approach was introduced for MgO-NPs using A. millefolium extracts in varying aqueous–ethanol ratios and demonstrated their promising antiplasmodial activity against P. falciparum. Among the tested formulations, the 30:70 (DW:EtOH) extract produced MgO-NPs with the strongest activity against the chloroquine-sensitive 3D7 strain (IC₅₀ = 0.17 mg/ml), comparable to that of chloroquine.
These findings highlight the potential of A. millefolium-based MgO-NPs as eco-friendly and effective candidates for developing alternative antimalarial therapies, particularly in the face of increasing drug resistance. Nonetheless, limitations such as the lack of in vivo validation and XRD-based crystallinity analysis should be addressed in future work. Further studies should also explore the mechanism of action and the possible broader-spectrum antimicrobial properties of these nanoparticles.
Acknowledgement :
We would like to express our gratitude to those who kindly facilitated this study, particularly Ms. Zahra Farzaneh for her technical assistance.
All parts of the current study were approved by Ethic Committee of Tehran University of Medical Sciences (Ethic ID: IR.TUMS.MEDICINE.REC.1402.650).
Funding: Research funding from Tehran University of Medical Sciences (Grant Number: 1402-4-99-6 9744) is gratefully acknowledged.
Conflict of Interest :
No potential conflict of interest is reported by the authors.
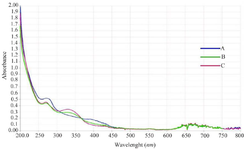
Figure 1. Ultraviolet-visible (UV-Vis) spectroscopy of green-synthesized MgO nanoparticles using the plant extract of Achillea millefolium, in three different proportions.
- A) 30D.W:70ETH, B) 50D.W:50ETH, and C) 70D.W:30ETH.
|

Figure 2. FTIR spectrum of three different proportions of A. millefolium extracts and synthesized MgO nanoparticles. A) 30D.W:70ETH, B) 50D.W:50ETH and C) 70D.W:30ETH.
|
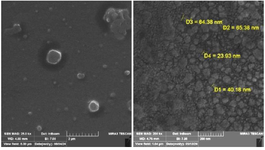
Figure 3. Fe SEM images of MgO nanoparticle.
|

Figure 4. Histogram of size distribution from the dynamic light scattering (DLS) analysis of biosynthesized Achillea millefolium MgO NPs at three different proportions. A) 30D.W:70ETH, B) 50D.W:50ETH, and C) 70D.W:30ETH.
|
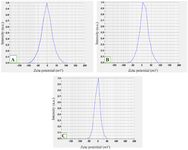
Figure 5. Zeta potential of Achillea millefolium MgO NPs. The value zeta potential of A.millefolium MgO NPs with three different proportions. A) 30D.W:70ETH, B) 50D.W:50ETH, and C) 70D.W:30ETH.
|

Figure 6. Inhibition percent of RBC hemolysis by Achillea millefolium extract and A. millefolium MgO NPs.
|
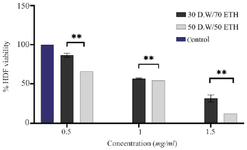
Figure 7. the MTT assay of MgO NPs with proportions of (30D.W:70ETH) and (50D.W:50ETH) at three different concentrations: 0.5 mg/ml, 1 mg/ml, and 1.5 mg/ml. The results of the Tukey multiple comparisons test indicate a statistically significant finding, at **p<0.01.
|
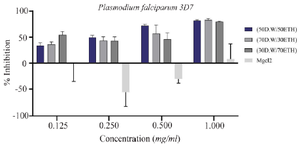
Figure 8. The growth-inhibitory effect of MgO NPs at three different proportions: (30 D.W:70 ETH), (50D.W:50 ETH), and (70 D.W:30 ETH), based on the Plasmodium falciparum strain 3D7 and MgCl2. Two-way ANOVA showed differences between proportions are not significant.
|
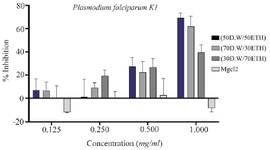
Figure 9. The growth-inhibitory effect of MgO NPs at three different proportions: (30D.W:70ETH), (50D.W:50ETH), and (70D.W: 30ETH), based on the Plasmodium falciparum strain K1 and MgCl2. Two-way ANOVA showed differences between proportions are not significant.
|
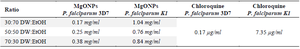
Table 1. Determination of MgO NPs and chloroquine IC50 on P. falciparum species
|
|