Expression and Purification of Functionally Active Recombinant Human Alpha 1-Antitrypsin in Methylotrophic Yeast Pichia Pastoris
-
Arjmand, Sareh
-
Department of Molecular Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran
-
National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
School of Pharmacy, Zanjan University of Medical Science, Zanjan, Iran
-
Bidram, Elham
-
Department of Clinical Biochemistry, Tarbiat Modares University, Tehran, Iran
-
 Sahebghadam Lotfi, Abbas
Ph.D., National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran - Karaj Highway, Tehran, Iran, Tel: +98 21 44580309 Fax: +98 21 44580399 E-mail: lotfi-ab@nigeb.ac.ir
Sahebghadam Lotfi, Abbas
Ph.D., National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran - Karaj Highway, Tehran, Iran, Tel: +98 21 44580309 Fax: +98 21 44580399 E-mail: lotfi-ab@nigeb.ac.ir
-
National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
Department of Clinical Biochemistry, Tarbiat Modares University, Tehran, Iran
-
Shamsara, Mehdi
-
National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran
-
Mowla, Seyed Javad
-
Department of Molecular Genetics, Faculty of Biological Sciences, Tarbiat Modares University, Tehran, Iran
Abstract: Human alpha 1-antitrypsin (AAT) cDNA was obtained from HepG2 cell lines. After PCR and construction of expression vector pPICZα-AAT, human AAT was expressed in the yeast Pichia pastoris (P.pastoris) in a secretary manner and under the control of inducible alcohol oxidase 1 (AOX1) promoter. The amount of AAT protein in medium was measured as 60 mg/l 72 hr after induction with methanol. Results indicated the presence of protease inhibitory function of the protein against elastase. Purification was done using His-tag affinity chromatography. Due to the different patterns of glycosylation in yeast and human, the recombinant AAT showed different SDS-PAGE patterns compared to that of serum-derived AAT while pI shifted from 4.9 in native AAT compared to 5.2 in recombinant AAT constructed in this study.
Introduction :
Human alpha 1-antitrypsin (AAT) is the major member of serin protease inhibitor (serpin) superfamily in plasma which is mainly produced in liver (1). It is now recognized that AAT is a potent inhibitor of multiple serine proteases, and protects tissues against their harmful effects. The average concentration of AAT in plasma is 1.3 mg/ml, with a half-life of 3 to 5 days. Individuals with plasma AAT values below 0.7 mg/ml are considered to be AAT deficient (2,3). Intravenous administration of a pasteurized pooled human plasma AAT product (Prolastin; Bayer Corporation) is used to increase AAT levels in deficient individuals
(4). This approach of therapy is practical and
feasible (5,6). However, there are two major obstacles; namely source limitation and risk for emerging viruses (7). Alternatively, the recombinant versions of AAT have been under intensive investigation.
Since the early 1980s, the human AAT gene has been expressed in various hosts, including Escherichia coli (E.coli), yeasts, insect cells, CHO cells, as well as in transgenic plants and animals. Protein size, glycosylation pattern, metastable inhibitory nature of AAT and production cost represent the challenges in the production of recombinant AAT (8-12).
We considered the metylotrophic yeast P.pastoris as an attractive host for production of human AAT. It is a single-cell eukaryote with the advantages of both prokaryotic and eukaryotic hosts. One of its main advantages is the ability for introducing many of post-translational modifications such as glycosylation and proteolytic processing which is obtained through moving in the secretory pathway (13). For glycoproteins such as AAT, these modifications are very important for appropriate function and/or structure (14,15).
In the present study, the AAT was expressed in P.pastoris as a fusion protein to a histidine tag (His-tag) which facilitates purification step. Signal sequence from Saccharomyces cerevisiae α-mating factor (α-MF) was included to direct secretion of the protein to extracellular medium. This powerful expression system made use of the highly inducible alcohol oxidase 1 (AOX1) promoter to express large quantities of glycosylated protein. The P.pastoris produced AAT activity and its features were evaluated for the first time using elastase inhibitory assay and Isoelectric Focusing (IEF), respectively. Mobility shift caused by addition of tunicamycin-a N-linked glycosylation inhibitor- to culture indicated the effect of glycosylation on the protein weight and SDS-PAGE protein band pattern. These data will be used in future studies for generating a new recombinant AAT protein with pharmaceutical objectives.
Materials and Methods :
Bacteria and P.pastoris strains and media
The E.coli strain DH5α was used for propagation of recombinant plasmids. The P. pastoris strain X-33 (Invitrogen) was used as a host for the protein expression. Recombinant bacteria were cultured in low salt LB broth medium (0.5% (w/v) yeast extract, 1% (w/v) tryptone, 0.5% (w/v) NaCl) supplemented with 25 µg ml-1 of zeocin (Invitrogen). P.pastoris was cultured on the following media: YPDS plates (1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) dextrose, 1 M sorbitol, and 1.5% (w/v) bacteriological agar) supplemented with 100 µg ml-1 of zeocin; Buffered Glycerol-complex Medium (BMGY) medium (1% (w/v) yeast extract, 2% (w/v) peptone, 100 mM potassium phosphate, 1.34% (w/v) Yeast Nitrogen Base (YNB), 4×10-5% (w/v) biotin, 1% (v/v) glycerol, pH=6.0); Buffered Methanol-complex Medium (BMMY) medium (1% (w/v) yeast extract, 2% (w/v) peptone, 100 mM potassium phosphate, 1.34% (w/v) YNB, 4×10-5% (w/v) biotin, 0.5% (v/v) methanol, pH=6.0) supplemented with 2% Phenyl Methyl Sulphonyl Fluoride (PMSF); Minimal Dextrose (MD) agar (1.34% (w/v) YNB, 4×10-5% (w/v) biotin, 2% (w/v) dextrose, 2% (w/v) agar); and Minimal Methanol (MM) agar (1.34% (w/v) YNB, 4×10-5% (w/v) biotin, 0.5% (v/v) methanol, 2% (w/v) agar).
Cloning of AAT in pPICZαB plasmid
Since the hepatic cells are the main source for AAT expression, total RNA was extracted from human hepatocellular carcinoma (Hep G2) cell line (obtained from the Pasteur Institute of Iran, Tehran). Briefly, about 107 cells were harvested and total RNA was isolated using RNeasy Mini Kit (Qiagen) according to manufacturer’s instruction. Primers 1 (5´CTCTCGAGAAAAGAGAGG CTGAAGCTGAAGATCCCCAGGGAGATG 3´) and 2 (5´ GCTACTCTAGATAATTTT TGGGTGGGATTCAC 3´) were employed to amplify AAT cDNA excluding its signal peptide (amino acid residues 1-24) from the 100 ng RNA in 20 µl reaction using the One-Step RT-PCR Kit (Qiagen). In designing the primers, restriction sites of XhoI and XbaI were incorporated at the 5’ ends of the PCR products. The cDNA product was cloned into the pPICZαB plasmid (Invitrogen) in a way to introduce the His-tag epitope at the carboxyl terminal of the recombinant AAT.
P.pastoris transformation and selection
P.pastoris cells were grown overnight in YPD broth (at 30 ○C/ 250 rpm) and prepared for transformation according to the manufacturer’s recommendations (Invitrogen). The recombinant plasmid was linearized with SacI and electrotransformed into the 80 µl of competent P.pastoris using a Bio-Rad gene-pulser apparatus (at 1.5 kV, 25 F, 400 Ω and 8 ms). Hundreds of transformed cells were selected by growing on YPD agar plate in presence of 100 µg ml-1 of zeocin.
Determination of methanol utilizing (Mut) phenotype
Transformation of P.pastoris X-33 cells with linearized DNA results in two phenotypes of host cells in respect with methanol utilization (Mut). Since single crossover recombination at the AOX1 promoter region is favored, most of the transformants have Mut+ phenotype in which the AOX1 gene is intact. However, due to the presence of the AOX1 terminator sequences in the plasmid, there is a chance for second recombination at this region which result in replacing of AOX1 gene with desired one hence creating MutS phenotype. The latter is weak in metabolizing methanol. The Mut phenotype for the transformed X-33 cells was determined by growing one hundred clones on minimal media with methanol (MM) or dextrose (MD) plates. Chromosomal integration of the plasmid DNA and right orientation of the expression cassette containing the AAT cDNA were characterized by PCR analysis and DNA sequencing. 5′ and 3′ AOX1 primers (5' GAC TGGTTCCAATTGACAAGC 3' and 5’ GC AAATGGCATTCTGACATCC 3'), directed to the AOX1 promoter and the transcription terminator were used for this purpose.
Shake flask protein expression
Ten verified transformants were grown at 30○C in 10 ml BMGY medium for 24 hr until reaching an OD600 of 10. The cells were harvested and resuspended in 50 ml BMMY medium containing 0.5% (v/v) methanol. After incubating the cells at 30ºC/200 rpm for 72 hr methanol was added at 1% (v/v) once a day to maintain induction.
SDS-PAGE and silver staining
The cells were removed and the supernatant was precipitated by using 100% Trichloroacetic Acid (TCA) solution. After drying, the pellet was resuspended in loading buffer containing β-mercaptoethanol, heated for 10 min, in boiling water and electrophoresed at 12% SDS-PAGE/100 V along with molecular weight marker (Fermentas). Gel was stained with silver nitrate according to Celis and coworkers (16).
Western blot analysis
Protein samples resolved on SDS-PAGE, were electro-blotted to Polyvinylidene Difluoride (PVDF) membrane (Millipore) in transferring buffer (0.025 M Tris, 0.19 M glycine, and 20% (v/v) methanol) overnight at 20 V/4°C. The membrane was treated with PBS-T-BSA (PBS, 0.1% (v/v) Tween 20, 1% (w/v) BSA) for 2 hr to block binding sites. After washing step, membrane was reacted with 1000-fold diluted goat anti-human alpha-1 antitrypsin polyclonal antibody, conjugated with HRP (Abcam) for 3 hr. To eliminate non-specific reactions, a supernatant of non-recombinant X-33 culture treated side by side accordingly as negative control. Subsequently, protein bands reacted positively, were visualized at the presence of 4-chloro1-naphtol substrate in PBS.
Quantification of AAT
The Enzyme-linked Immunosorbent Assay (ELISA) was used for quantitative determination of secreted AAT levels in the medium using human alpha 1-antitrypsin ELISA Quantitation Kit (GenWay Biotech, Inc) according to the manufacturer's recommendations. For all experiments the samples were diluted 200 folds in sample/conjugate diluents (50 mM Tris, 0.14 M NaCl, 1% (w/v) BSA, 0.05% (v/v) Tween 20, pH=8.0). Different concentrations of commercial human AAT (Sigma) were used to construct standard curve, while supernatant of non-recombinant P.pastoris (X-33 strain) culture was used as a negative control.
Affinity chromatography purification
For the purification of His-tag fused AAT, the supernatant was applied to a nickel-immobilized chelating sepharose fast flow column (Amersham, Biosciences). For this purpose supernatant first was diluted with equal volume of 2X binding buffer (50 mM NaH2PO4, 500 mM NaCl, 10 mM imidazole, pH=7.4) and loaded on to the column. After passing the wash buffer (50 mM NaH2PO4, 500 mM NaCl, a gradient of imidazole from 20 to 40 mM, and 0.05% (v/v) Tween 20, pH=7.4) through the column, the resin-bounded recombinant AAT was eluted with elution buffer (50 mM NaH2PO4, 500 mM NaCl, 250 mM imidazole, and 0.05% (v/v) Tween 20, pH=7.4).
Inhibitory activity assay
Elastase activity was measured by EnzChek® Elastase Assay Kit (Molecular Probes, Inc.) according to the manufacturer’s recommendations. The EnzChek kit contains DQ™ elastin soluble bovine neck ligament elastin that has been labeled with BODIPY® FL dye such that the conjugate’s fluorescence is quenched. The non-fluorescent substrate can be digested by elastase or other proteases to yield highly fluorescent fragments. The presence of an inhibitor such as AAT blocks the substrate digestion hence subsequent fluorescent emission. The resulting change in fluorescence level was monitored using a standard fluorometer (Hitachi F-3010) with a maximum absorption at 505 nm and a maximum fluorescence emission at 515 nm. Commercial human AAT was used as a positive control and the elution buffer and the supernatant from non-recombinant P.pastoris (X-33 strain) culture as negative controls.
Isoelectrofocusing (IEF)
IEF was accomplished on a pharmacia flatbed electrophoresis apparatus at 4°C using high voltage and carrier pharmalytes (Sigma) over the pH range of 4.5-5.4. The experiment was performed on the 7% polyacrylamide gradient gel. Subsequently, the IEF gel was stained with coomassie brilliant blue R-250.
Inhibiting protein glycosylation using tunicamycin
Tunicamycin was used to block in vivo glycosylation. To determine differences in glycosylation of the recombinant protein, tunicamycin (Sigma, T7765) was used at final concentration of 2.5 µg/ml in BMMY culture medium. Cell density was checked every day and after 96 hr of induction with methanol, the supernatant was precipitated using 100% TCA solution. The results of SDS–PAGE and western blotting were compared to those of P.pastoris at the same conditions except no tunicamycin.
Results :
AAT cloning in P.pastoris
The cDNA of AAT from HepG2 cell line was cloned in frame with the α-MF secretion signal into the expression vector pPICzαB. The resulting plasmid was named pPICZα-AAT. Transformants were selected on YPDS agar containing zeocin, and the Mut phenotype was determined by growing them on selective MD and MM media. According to corresponding data, more than 90% of transformants had equal growth features on both media that is a proof for Mut+ phenotype. PCR results with AOX1 primers for genomic DNA were consistent with those of screening in selective media and confirmed that the AAT gene had been integrated into the AOX1 locus. The correct orientation and nucleotide sequence of AAT cDNA within the P.pastoris genome verified by sequencing. Mut+ clones showed two different bands in agarose gel electrophoresis, the 1.8 kb fragment which was amplified from AAT expression cassette flanked by AOX1 sequences, and another 2.2 kb fragment which was amplified from AOX1 gene of P.pastoris. In MutS clones, only 1.8 kb band was observed, which demonstrates the disruption of AOX1 gene (Figure 1).
SDS-PAGE and AAT purification
Supernatant samples from cultures of few positive clones and one negative clone (non-recombinant X-33) were subjected to SDS-PAGE analysis after precipitation with TCA. The results provided an additional band near 60 kDa only in positive clones (Figure 2, Lanes 1-6). The secreted protein was purified from the supernatant by passing through a nickel column and fractions were screened by SDS-PAGE. The purified protein samples were seen as two or three smeared bands after Coomassie brilliant blue R-250 staining (Figure 3A).
Immunoblot assays
To confirm the identity of recombinant protein, the purified protein was reacted with AAT antibody in a western blot analysis. The recombinant AAT was identified as two distinct bands (Figure 3B, Lane 2) with a molecular weight slightly larger than that of the commercial protein (Figure 3B, Lane 1).
AAT levels in supernatant were determined by quantitative ELISA, using two specific monoclonal antibodies against AAT. Level of protein expression was determined in ten Mut+, three Muts, and one non-recombinant X-33 clones. According to our data, methanol induction at concentration of 0.5% (v/v) for the first day and 1% (v/v) for the next two days could result in highest level of protein secretion in Mut+ phenotype. Average quantities of protein expression were 60 mg/l and 44 mg/l after 72 hr for Mut+ and Muts clones, respectively (Figure 4).
Functional assay and IEF
Data from the activity assays provided an increase in detected fluorescence levels when the elution buffer and/or the supernatant of non-recombinant X-33 culture were screened. However, no changes were seen for commercial AAT and the recombinant AAT (Figure 5).
After staining with Coomassie brilliant dye, recombinant AAT protein was visualized as three bands in IEF acrylamide gel migrating at 5.1 to 5.2 kDa, while plasma derived AAT was detected as five bands from 4.7 to 4.9 kDa (data was not shown).
Effect of tunicamycin
To investigate whether the protein bands with higher molecular weight were due to different glycosylation, we expressed the AAT in media containing tunicamycin, which blocks glycosylation in vivo, and analysed the supernatant by SDS-PAGE and western blotting. In the presence of tunicamycin the AAT band shifted to a position lowers than that of natural and recombinant proteins (Figure 6).
Discussion :
The methylotrophic yeast P.pastoris, has emerged as an important and efficient host in modern biotechnology. It has been used as a successful expression system for recombinant protein in academic research and industrial production (17,18). A large variety of proteins that cannot be expressed in E.coli at the correct level of post-translational maturation have been subsequently produced in the eukaryotic cells (19,20). The glycosylation pattern in P.pastoris is different from that of mammalians and this may or may not be a problem depending on the target protein and its application.
AAT is a glycoprotein and addition of carbohydrate moieties to appropriate positions is an important post-translational modification that is essential for the stability of AAT protein (21,22). Therefore, to obtain proper glycosylation is one of the major concerns in the production of recombinant AAT. The most suitable candidates for production of AAT in correct form with closest modification to authentic human AAT are mammalian cells (7). But using these cells for production of recombinant protein is a time consuming process and its industrial/ pharmaceutical application is not cost efficient. Using yeasts like P.pastoris for production of AAT glycoprotein seems to be an effective alternative and has resulted higher yields. P.pastoris in comparison with two other yeasts (Saccharomyces cerevisiae and Hansenula polymorpha) produces recombinant AAT with the glycosylation pattern closer to that of the authentic human AAT (23). Furthermore, P.pastoris core oligosaccharides were reported to have no immunogenic terminal α-1,3 glycan linkages (24). We selected metylotrophic yeast P.pastoris for production of AAT and according to our data this system could potentially be used as an effective host for this purpose.
The AAT was produced in quantities of up to 60 mg/l in shake flask. In SDS-PAGE, our recombinant protein showed a different migration pattern in comparison to that of plasma AAT. This could be the result of different patterns of glycosylation. Tunicamycin treatment which inhibits the glycosylation of secretary proteins, confirmed this idea. These differences in glycosylation pattern affected the mobility of recombinant protein on IEF and increased its pI in comparison to plasma derived AAT from 4.2-4.9 (25,26) to 5.1-5.2.
Conclusion :
Activity assay demonstrated the protease inhibitory power for this recombinant protein. The high mannose glycosylation apparently did not alter the main function of the protein. Besides, no change on AAT secretion was observed. The immunogenic properties of this protein will be studied for its medicinal potential. Further studies are needed to screen protein expression while increasing the production scale.
Acknowledgement :
This work was supported by International Center for Genetic Engineering and Biotechnology (ICGEB) Trieste grant. We are also thankful to Dr. Mirfakhraee for his comment.
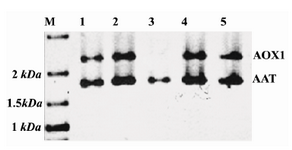
Figure 1. PCR with AOX1 primers from recombinant P.pastoris genome. Lanes 1, 2, 4 and 5 belong to Mut+ clones showing two different bands in the gel. The 1.8 kb band was amplified from AAT expression cassette flanked by AOX1 sequences, and the 2.2 kb band was amplified from AOX1 gene of P.pastoris. Lane 3 belongs to a MutS clone where only the 1.8 kb band is seen. This confirms that AOX1 gene has been disrupted in these cells
|
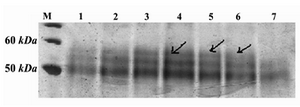
Figure 2. SDS-PAGE and silver staining of supernatants of positive clones. M) protein molecular weight marker; Lanes 1-6: supernatants of positive clones which show an extra band in ~60=kDa; Lane 7: supernatant of the non-recombinant X-33 clone, used as negative control
|
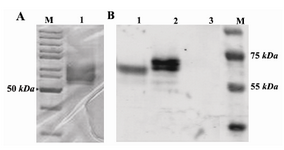
Figure 3. A) SDS-PAGE analysis of protein purification with nickel column stained with Coomassie brilliant blue. M: protein ladder; Lane1: nickel column-purified protein. B) Western blotting of AAT protein on PVDF membrane. M: protein ladder; Lane 1: commercial plasma derived AAT; Lane 2: nickel column-purified recombinant AAT protein; Lane 3: supernatant of non-recombinant X-33 culture
|
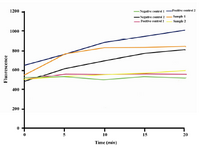
Figure 4. Comparison of quantities for secreted recom-binant AAT in Mut+, Muts and non-recombinant X-33 clones where identified by human alpha 1-antitrypsin ELISA Quantitation Kit in three consecutive days. Data represent the average of three independent experiments
|
Figure 5. Activity assay of the recombinant AAT Com-mercial plasma-derived AAT were used in 10 and 20 µg concentrations as positive controls 1 and 2; supernatant of non-recombinant X-33 and elution buffer were used as negative controls 1 and 2, respectively; 20 µg of the recombinant AAT from two different clones were used for inhibitory activity analysis. Active AAT inhibited increasing of fluorescence level
|
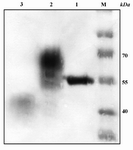
Figure 6. Expression of AAT protein in the absence and presence of tunicamycin monitored by western blot analysis. M: protein ladder; Lane 1: commercial plasma-derived AAT; Lane 2: control recombinant AAT protein; Lane 3: recombinant AAT produced in culture containing 2.5 µg/ml tunicamycin. Weakness of this band is due to inhibitory effects of tunicamycin for yeast growth with subsequently lowers cell density and secreted proteins in the medium
|
|