Regulation Studies of Telomerase Gene in Cancer Cells by Lentinan
-
 Sreenivasulu, Kamma
Ph.D., Department of Biotechnology, KL University, Vaddeswaram, India, E-mail: sathwik.kamma@gmail.com
Sreenivasulu, Kamma
Ph.D., Department of Biotechnology, KL University, Vaddeswaram, India, E-mail: sathwik.kamma@gmail.com
-
Department of Biotechnology, KL University, Vijayawada. A.P., India
-
Vijayalakshmi, Muvva
-
Department of Botany and Microbiology, Acharya Nagarjuna University, Guntur, India
Abstract: Lentinan a polysaccharide from medicinal mushroom i.e Lentinus, has been known to have anticancer properties. Telomerase activity is not observed in normal healthy cells, whereas in cancerous cells telomerase expression is high. Telomerase represents a promising cancer therapeutic target. We investigated the inhibitory effect of lentinan on telomerase reverse transcriptase gene (hTERT) which is essential for telomerase activity. To assess the transcriptional effect, DLD -1 cancer cells were cultured in the presence of various concentrations of lentinan. TRAP assay, RT-PCR analysis were performed to find telomerase activity and hTERT gene expression respectively. Since C-myc is known to regulate hTERT, expression of C-myc was also determined. Culturing cells with lentinan resulted in down regulation of hTERT and C-myc expression.These results indicate that lentinan inhibits telomerase activity by down regulating hTERT expression via suppression of C-myc in cancer cells.
Introduction :
Human telomerase is a ribonucleoprotein that adds TTAGGG repeats to telomere ends (1). Telomerase activity is not detectable in normal cells, with exception of germ cells and renewal tissues. However, it is present in
80-90% of human cancer specimens (2). Telomerase comprises three major components i.e htr (human telomerase RNA component), tp1 (telomerase associate protein) and hTERT (human telomerase reverse transcriptase) (3). Amongst these three components, hTERT plays a key role in telomerase activation. Of the possible regulators C-myc is known to down regulate the expression of hTERT. Telomerase inhibitors can be divided into two groups (4).
One group binds to telomerase and blocks its activity, whereas others are suppressors of mRNA expression of hTERT resulting in altered telomerase activity (5).
Lentinan is a ß-glucan with a glycosidic ß-1,3:ß-1,6 linkage (Figure 1). It is an anti-tumor polysaccharide from the shiitake (Lentinulaedodes) mushroom. Lentinan is a polysaccharide which is free of nitrogen, and has a molecular weight of approximately 500,000 Da. The Japanese pharmaceutical company Ajinomoto developed Lentinan, which is an intravenously administered anti-cancer agent. Lentinan is one of the host-mediated anti-cancer drugs which has been shown to affect host defense immune systems (6). Limited clinical studies of cancer patients have associated le ntinan with a higher survival rate, higher quality of life, and lower re-occurrence of cancer (7).
The aim of present study is to determine the effect of lentinan on gene expression regulation of hTERT and C-myc.
Materials and Methods :
Lentinan was isolated from lentinusedodes (Yap and Ng, 2001). DLD-1 gastric cancer cell lines were cultured in RPMI-1640 medium containing freshly prepared DMSO solution of lentinan at various concentrations (0,2,4,6,8,10 ug/ml). The final concentration of DMSO in the medium is 0.1% (v/v). Control cells were grown in medium supplemented with 0.1% DMSO.
Cytotoxicity of cell was evaluated by MTT assay which is based on the conversion of MTT to MTT-formazan by mitochondrial enzymes as previously described (8).
Telomerase activity
Telomerase activity was measured by PCR based TRAP as previously described (9) using TRAPEZE ELISA telomerase detection kit based protocol (Chemicon, CA, USA).
The brief procedure is as follows: 5×105 cells per well were seeded in 6-well plate and incubated with lentinan mixture at the above specified concentrations for 48 hrs at 37 ?C. The cell pellet was then cooled on ice, lysed with CHAPS lysis (10 mMTris–HCl (pH=7.5), 1 mM MgCl2, 1 mM EDTA, 0.1 mM phenylmethyl sulfonylfluoride, 5 mM -mercaptoethanol, 0.5% (3-(3-cholamidopropyl) dimethylamino-1- propanesulfonate, and 10% glycerol) buffer for 30 min and centrifuged at 12,000× g at
4 ºC for 20 min. The TRAP PCR reaction mixture contains 1 µg of protein from each cell extraction, a mixture of biotinylated TS primer, RP primer, internal control (K1primer and TSK1 template), 2.5 mMdNTPs and 2 units Taq DNA polymerase. The PCR was performed for 33 cycles at 94 ºC for 30 sec, 55 ºC for 30 sec, 72 ºC for 30 sec and followed by final extension at 72 ºC for 10 min. The PCR product was separated for determining the degree of telomeric repeats (by 10% non-denaturing polyacrylamide gel electrophoresis) and the telomerase level (by ELISA detection) as described below.
Gene expression of hTERT, C-myc and ?-actin
After cultivation, total RNA was isolated from cells with total RNA extraction kit (qiagen). RT-PCR was performed as per kit (bio-rad) as per manufactures instructions using following primer (Table 1).
The PCR conditions were as follows: for hTERT and ?-actin 35 cycles of denaturation at 95 oC for 30 sec, annealing at 59.2 oC for 30 sec and extension at 72 oC for 30 sec, respectively.
For C-myc, ? -actin 30 cycles of denaturation at 95 oC for 30 sec and extension at 72 oC for 30 sec, respectively. PCR products were loaded on 0.8% agarose gel containing ethidiumbromide. The product bands were analysed using gene snap software (Syngene, NJ, USA). The relative intensity was calculated by normalizing with ß actin.
Result :
By MTT assay the percentage of viability was calculated by defining the absorption of cells without lentinan treatment as 100 percent (Figure 2) at 10 microlitre concentration; also cell viability was observed above 75%.
DLD-1 cells were treated with lentinan at concentration 0,2,4,6,8,10 ug/ml. Telomerase activity was examined by TRAP assay. Decreased telomerase level was interpreted by reduction or disappearance of bands (Figure 3). Telomerase activity was determined by TRAPEZE-ELISA and levels were expressed as % relative activity (Figure 4).
DLD-1cells were incubated for 48 hrs at lentinan concentrations of 0,2,4,6,8,10 ug/ml. Effect on gene expression was examined by RT-PCR (Figures 5 and 6). Percentage of band intensities was calculated with the aid of genesnap software (Tables 2 and 3). ß actin is internal control of gene expression.
To evaluate the mechanism for telomerase inhibition by lentinan we investigated the effect of hTERT expression in DLD 1 cells using RT-PCR technique. For this, we treated DLD-1 cells with lentinan at concentration 0,2,4,6,8,10 ug/ml and then examined the telomerase activity by TRAP assay. We observed a decrease in telomerase level by reduction or disappearance of bands (Figure 4) Percentage of band intensities was calculated with the aid of genesnap software (Tables 2 and 3) ?-actin is internal control of gene expression. Lentinan clearly inhibited the expression of hTERT mRNA in dose dependent manner, indicating that telomerase activity is modulated at the transcriptional level. Since C-myc is a known regulator of hTERT we investigated and confirmed that lentinan reduced the expression levels of C-myc mRNA and hTERT expression (Figure 7). These observations suggest that lentinan decreases the telomerase activity by down regulating the hTERT expression via cmyc
Discussion :
Since most cancer cells posses telomerase activity, one probable advantage of telomerase targeted therapy would be its specificity on telomerase positive tumour cells, because most human somatic tissues are telomerase negative. On the basis of these observations various types of telomerase inhibitors have been discovered and developed. Such inhibitors include hTR antigens oligonucleotides (2’-0-methyl RNA and peptide nucleic acids). (9) Reverse transcriptase inhibitors (ex: 3’ azido 3’ deoxythymidine) (10) and natural products (ex.telomestatin and sulfoquinovosyldiacyl glycerol) (11). These inhibitors directly inhibit telomerase activity. Modulators of mRNA expression of telomeral components (i.ehtert) have been regarded as another type of anti telomeral agents. These include all transretonoic acid (12), 5, 6 –trans- 16 -ene - vitamin D3 (13) ceramide (14) and curcumin (15). These compounds act as supressors of hTERT mRNA expression which results in altered telomerase activity.
Conclusion :
These studies provide support for role of lentinan as chemopreventative agent against cancer cells. Chemoprevention as a scienti?c ?eld, may be considered still at its infancy, and includes the use of natural or pharmacological agents to suppress, arrest or reverse carcinogenesis at its early stages. Natural products like genistein, resveratrol, curcumin, retinoic acid and epigallocatechin-3-gallate are proved as chemopreventive agents.
Acknowledgement :
Authors are very much thankful to management of KL University, Vijayawada for their encouragement and constant support throughout this research work.
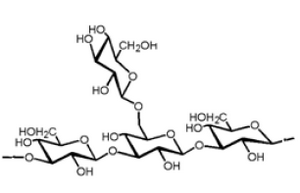
Figure 1. Lentinan beta glucan structure
|
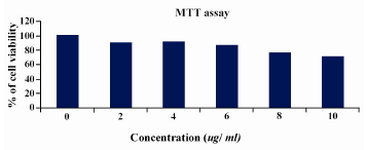
Figure 2. MTT ASSAY cell lines were treated with various concentrations of lentinan and cell viability was determined
|
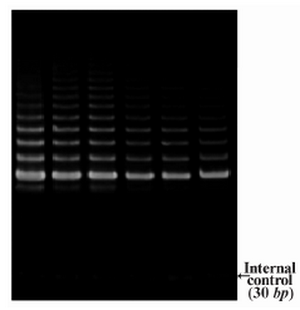
Figure 3. Telomerase activity by TRAP assay
|
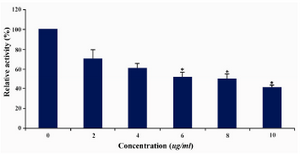
Figure 4. Telomerase levels estimation by TRAPEZE-ELISA
|
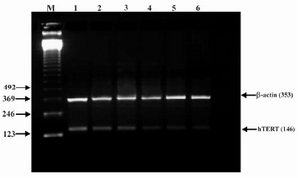
Figure 5. Effect of lentinan on hTERT gene expression (Lanes 1 through 6 represent 0, 2, 4, 6, 8 and 10 ug/ml lentinan concentrations at which DLD-1 gastric cancer cell lines were incubated; M is molecular weight marker)
|
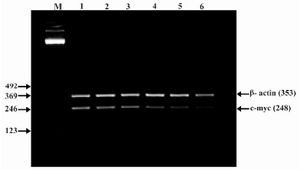
Figure 6. Effect of lentinan on C-myc gene expression (Lanes 1 through 6 represent 0, 2, 4, 6, 8 and 10 ug/ml lentinan concentrations at which DLD-1 gastric cancer cell lines were incubated; M is molecular weight marker)
|
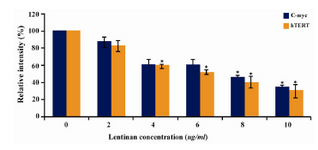
Figure 7. Effect of lentinan on hTERT and C-myc gene expression
|
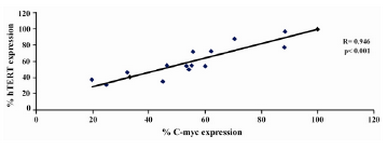
Figure 8. Graph showing correlation of hTERT expression and C-myc expression in DLD-1 cells after lentinan incubation (genesnap software)
|
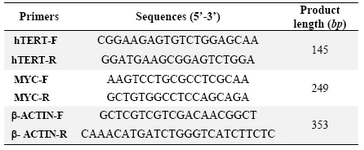
Table 1. List of primers
|
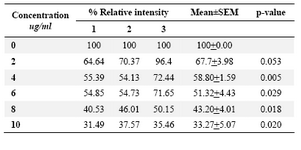
Table 2. Relative band intensity of hTERT gene expression in DLD-1 cells.Results are the average of three independent experiments (genesnap software)
|
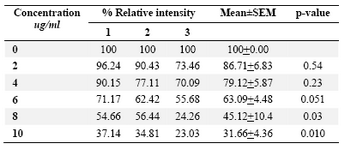
Table 3. Relative band intensity of C-myc gene expression in DLD-1 cells. Results are the average of three independent
|
|