Monoclonal Antibody Against Sortilin Induces Apoptosis in Human Breast Cancer Cells
-
Simonian, Miganoosh
-
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Haji Ghaffari, Mozhan
-
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Salimi, Ali
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Mirzadegan, Ebrahim
-
Nanobiotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sadeghi, Niloufar
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Ebrahimnezhad, Nasim
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Fazli, Ghazaleh
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Fatemi, Ramina
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Bayat , Ali Ahmad
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Milani, Saeideh
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
 Negahdari, Babak
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 9126571037, 21 22432020 Fax: +98 21 22432021 E-mail: negahdari_md@yahoo.com
Negahdari, Babak
Department of Medical Biotechnology, School of Advanced Technologies in Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 9126571037, 21 22432020 Fax: +98 21 22432021 E-mail: negahdari_md@yahoo.com
-
 Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 9126571037, 21 22432020 Fax: +98 21 22432021; E-mail: rabbani@ari.ir
Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 9126571037, 21 22432020 Fax: +98 21 22432021; E-mail: rabbani@ari.ir
Abstract: Background: Sortilin has an important role in various malignances and can be used as a promising target to eradicate cancer cells.
Methods: In this study, the expression of sortilin in 4T1 and MDA-MB231 cell lines was evaluated by flow cytometry and immunocytochemistry. Apoptosis assay was also applied to evaluate apoptosis induction in 4T1 and MDA-MB231 cell lines.
Results: Based on cell surface flow cytometry results, anti-sortilin (2D8-E3) mAb could recognize sortilin molecules in 79.2% and 90.3% of 4T1 and MDA-MB231 cell-lines, respectively. The immunocytochemistry staining results confirmed sortilin surface expression. Apoptosis assay indicated that anti-sortilin mAb could induce apoptosis in 4T1 and MDA-MB231 cell lines.
Conclusion: Our study revealed the important role of surface sortilin in breast carcinoma cell survival and its possible application as a therapeutic agent in cancer targeted therapies.
Introduction :
Breast cancer is among the most common cancers especially in women worldwide 1. In the Middle East, breast cancer is the most common malignancy among women. Similarly, in our country, Iran, the incidence of breast cancer is estimated at 22.4 (summary of report on cancer incidence in Iran, 2000) per 100,000 women. Although chemotherapy combined with surgical excision is the mainstay of treatment for patients with breast cancer 2, the frequently encountered resistance to chemotherapeutic drugs is a crucial factor in breast cancer recurrence and metastasis that subsequently leads to high mortality rates 3.
Currently, monoclonal antibodies (mAbs) are broadly utilized for clinical diagnosis and treatment of cancers. Albeit antibody-based therapeutic strategy in solid tumors is well established 4-6, the same is yet to be fully developed in the context of breast cancer.
The expression of proteins related to the nervous system in cancer is an attractive feature of several carcinomas that may be due to the common developmental basis of neurons and epithelial cells, and both of them are from the neuroepithelial layer of the embryo 7-9. Sortilin, a neuronal type-1 membrane protein, is encoded by the SORT1 gene on chromosome 1 10. This type I membrane glycoprotein is a part of mammalian Vacuolar Protein Sorting 10 protein (VPS10P) family of receptors that are highly expressed in both central and peripheral nervous systems 11. The crystal structure of sortilin reveals that the sortilin ectodomain in complex with neurotensin shapes a ten-bladed beta-propeller structure with an inner tunnel that comprises multiple ligand binding sites 12, and is largely involved in protein transfer through a complex pattern between the endosome, lysosome, Golgi apparatus, and plasma membrane, leading to its contributions in multiple, qualitatively different biological processes including lipid and glucose metabolism besides neural development, survival, and cell death 13-17.
Besides its role as a regulator of neuronal viability, sortilin may also attribute to cancer pathogenesis. Involvement of the sortilin 1 receptor and its ligand, NT3 in prostate, colon, and pancreas tumors cell growth has been elucidated before 18. Indeed, remarkable increase of sortilin has been reported in human cancerous epithelial cells as compared to normal epithelial tissues 19.
It appears that sortilin participates in the progression of HT29 colon cancer cells by regulation of brain-derived growth factor, via interacting with its tyrosine kinase receptor TrkB 20. In A549 lung cancer cell line, sortilin induces the release and transfer of exosomes 21 and contributes to tumor cell adhesion and invasion in breast cancer cells 19. Furthermore, sortilin promotes prostate cancer cell growth through progranulin activity regulation 22. Additionally, sortilin has been identified as a co-receptor for pro-nerve growth factor (proNGF), and acts in complex with the neurotrophin receptor p75NTR to induce melanoma cancer cell invasion 23.
In spite of its strong relationship with cancer progression, sortilin has been shown to express in few cancer cell lines. In the investigation presented here, the expression of sortilin, using anti-sortilin monoclonal antibody (Padza Co., Iran) targeting extracellular domain in triple-negative MDA-MB231 and 4T1 breast cancer cells was examined and its inhibitory effects on tumor cell growth were evaluated in vitro.
Materials and Methods :
Cell line and culture conditions: The breast carcinoma cell lines, 4T1 and MDA-MB231(National Cell Bank of Iran), were cultured in their optimal conditions in RPMI-1640 (Gibco, Scotland), comprising 10% FBS (Gibco, Scotland), 100 units/ml penicillin (ICN Biomedicals, USA), and 100 μg/ml streptomycin (Sigma-Aldrich, USA) at 37°C in a humidified incubator with 5% CO2 atmosphere.
Detection of sortilin cell surface expression by flow cytometry: Cell lines were cultured in tissue culture flask. After 24 hr, when cells reached 70-80% confluency, they were detached by 0.25% trypsin-EDTA (Gibco, Scotland) and washed three times with cold Phosphate Buffered Saline (PBS), transferred to flow cytometry tubes and blocked with 5% sheep serum for 30 min at 4°C. Then, cells were incubated with 10 μg/ml anti-sortilin monoclonal antibody clone 2D8-E3 for 1 hr at 4°C (Padza Co, Iran). After 1 hr, the cells were washed with PBS containing 0.05% BSA and 0.1% NaN3, and the bound antibodies were detected by incubating the cells with a fluorescein isothiocyanate (FITC)–labeled sheep anti–mouse IgG (Padza Co, Iran) (1:50) at 4°C for 30 min in a dark place. The results were examined by a PAS III flow cytometer (Partec GmbH, Germany) and analyzed with FlowJo software (v.10). The calculation of average FITC intensities was carried out by multiplication of the Percentage of Positive cells (POP) to Mean Fluorescence Intensity (MFI) (POP×MFI).
Immunocytochemistry (ICC): After 24 hr of incubation at 37°C in a humidified incubator, cells were transferred to slides using Cytospin 4 Cytocentrifuge (Thermo Scientific, USA) (400 rpm, 5 min) at a density of 1×105 cells and fixed in neutral buffered formalin (NBF) for 10 min. The slides were washed three times (3×5 min) with Phosphate-Buffered Saline (PBS) (pH=7.4). The slides were blocked with 10% sheep serum containing 3% Bovine Serum Albumin (BSA) and 0.01% PBS-Tween 20 for 30 min at room temperature (RT). Slides were stained using primary antibody sortilin (Padza Co, Iran) at 10 μg/ml in 1% BSA+ 0.01% PBS-Tween 20 and the cells were incubated for 1 hr and 30 min in a humidity chamber at room temperature. After washing three times, the secondary sheep anti-mouse FITC-labeled antibody was added and incubated for 45 min at room temperature. After washing a further three times, the cell nuclei were stained with 4',6-diamidino-2-phenylindole dihydrochloride (DAPI) (Calbiochem, USA) at 1 μg/ml concentration for 10 min. Cells stained without primary antibody were utilized as negative control. Finally, after washing with PBS, the slides were mounted in 50% PBS-glycerol. Images were captured using a fluorescence microscope (Olympus, Japan).
Study of apoptosis: Apoptosis in 4T1, MDA-MB231, and control cells treated with sortilin Ab was measured at three different time points (24, 48, and 72 hr after treatment). Cells were scraped and washed in PBS (2x). After centrifuge, pelleted cells were incubated with 1×binding buffer containing FITC-annexin V (Padza Co, Iran) and stored in dark for 1 hr. Then, the cells were washed twice and Propidium Iodide (PI) was added. Finally, apoptotic cells were examined using a BD FACSLyric™ Flow Cytometer (BD Life Sciences, USA) and analyzed using the FlowJo software (V10).
Results :
Cell surface expression by flow cytometry and ICC: The expression of sortilin in 4T1, MDA-MB231, and Human Fetal Foreskin Fibroblast (HFFF) (As negative control) was investigated by flow cytometry. The 2D8-E3 mAb recognized sortilin molecules in 79.2% and 90.3% of 4T1 and MDA-MB231 cells while the expression of sortilin in HFFF was 2.1% as shown in figure 1. The average of FITC intensity (MFI×POP) values were 799.92 in 4T1, 517.42 in MDA-MB231, and 5.52 in HFFF (Figure 1, Table 1). For further confirmation, cell surface staining of 4T1 and MDA-MB231 cell lines was performed. It was demonstrated that they were expressing sortilin on their surfaces. The sortilin Ab indicated binding to cancerous cells at a concentration of 10 μg/ml (Figure 2).
Apoptosis induction by sortilin Ab: Next, the effect of sortilin Ab on survival of 4T1 and MDA-MB231 cells was measured by FACS analysis of annexin V staining 24, 48, and 72 hr post-induction. In each experiment, the percentage of apoptotic cells was examined as the sum of percentages of cells in early (i.e., annexin V positive PI-negative) and late (i.e., annexin V-positive, PI-positive) stages of apoptosis and was subtracted from negative control cells. The results showed that incubation with anti-sortilin antibody induced 8.45 early and 4.66% late apoptosis in 4T1 cells 24 hr after induction. This amount of apoptosis was markedly higher in 48 hr (73.3 early and 6.43% late apoptosis). In 72 hr, 25.6 and 23.3% of cells underwent early and late apoptosis, respectively (Figure 3A).
The results of 24 hr of incubation demonstrated that 2D8-E3 mAb could induce apoptosis in MDA-MB231 cell line with 12.3 and 8.11% of the cells undergoing early and late apoptosis, respectively. The values for 48 hr of incubation were 20.3% and 39.7% for early and late apoptosis. Moreover, 72 hr results for early and late apoptosis rates were 5.06 and 6.1%, respectively (Figure 3B). Also, it is obvious that the level of apoptosis increased over time after antibody induction in both cell lines. As compared to control cells, the 2D8-E3-treated cells showed 10.31 (24 hr), 28.92 (48 hr), and 8.95% (72 hr) apoptosis rates (Figure 3C), respectively. Furthermore, the isotype control mAb did not induce significant apoptosis in all examined cells.
Discussion :
Breast cancer accounts for 23% of all cancers diagnosed worldwide 2. Conventional cancer therapies are the gold standard treatment for cancers. Scientists have been looking for novel strategies with increased efficacies and more durable response in cancer patients. Although immunotherapy opened a new era in cancer treatment, it is faced with limited therapeutic effectiveness and life-threatening adverse reactions 24. Hence, there is an urgent need for finding new targets for improving immunotherapeutic effects.
This study is the first to report sortilin overexpression in 4T1 (murine breast cancer cell line) and MDA-MB231 (human breast cancer cell line) by means of flow cytometry. Our results indicate an ectopic expression of sortilin on the surface of breast cancer cells in comparison with HFFF as normal control cells and subsequent ICC analysis confirmed this finding. In terms of gene expression, sortilin mRNA abundance has been reported not only in breast but also prostate, colon, and pancreatic cancer cell lines 25. Human renal carcinoma 786-O and ACHN cells overexpress sortilin at mRNA and protein levels and sortilin is present in TrkB/p75NTR complex in RCC cells but its function is not associated with cell apoptosis induction or survival 26. On the other hand, sortilin in complex with two receptors containing a tyrosine kinase domain, TrkB and EGFR, might have an important role in angiogenesis and cell survival by induction of this pathway in Human Umbilical Vein Endothelial cells (HUVECs) exposed to sortilin-containing exosomes. Wilson et al proved that the angiogenesis process is impaired in A549 cells, a lung cancer cell line, in case of sortilin downregulation in exosomes 21. Also, sortilin in complex with TrkA, another tyrosine kinase receptor for NGF, mediates aggressiveness of breast cancer and is associated with tumor cell adhesion and invasion. The impact of sortilin in lymph node invasion of breast tumor cells stems from the fact that sortilin 1 as a type I receptor is an essential part of a receptor complex for pro-NGF induced neural cell death 16. In another study, the cleavage of luminal part of sortilin by matrix metalloproteinases produced soluble Sortilin (sSortilin) which activates the focal adhesion kinase pathway and regulates the downstream pathways involving either cell migration or metastasis in HT29 colorectal cancer cell line 27. Furthermore, sortilin enhanced invasive capacity of glioblastoma through GSK-3β/β-catenin/ twist pathway 28.
Considering the fact that sortilin can serve as an essential regulator of cell growth, according to above findings, there is no doubt that sortilin affects the incidence and growth of different types of tumor cells, so it could be an efficient target for cancer eradication. Recently, scientists exploited peptides conjugated with anticancer drugs to eradicate tumor cells. In a study carried out by Annabi et al, it was shown that a novel curcumin-peptide conjugate called TH1901 inhibited cancer cell proliferation in sortilin-positive cancers including ovary (ES2), breast (MD-MB231, HCC-1569, and HCC-1954), skin (SK-MEL-28 and A-375), and colorectal (HT-29) cancers 29. Two other peptides conjugated to docetaxel (TH1902) and doxorubicin (TH1904) are specifically designed to target sortilin receptor (SORT1). The former showed anti-proliferative and anti-migratory activities in MDA-MB-231 cells and caused tumor regression in murine MDA-MB-231 xenograft tumor model 30. Also, TH1904 inhibits the formation of 3D-tubular structures in an in vitro model of ovarian and triple negative breast cancer cells. These 3D-tubular structures are strongly associated with ES-2 ovarian cancer and MDA-MB-231 TNBC cells progression and invasion; hence, interference with their formation by targeting SORT-1 has potent therapeutic effects in cancer treatment 31. Moreover, sortilin receptor targeted by siRNAs abolishes the stem cell propagation, both in in vitro and in vivo breast cancer models, and decreases either the aggressiveness or metastatic state of tumor cells 32.
Although majority of investigators mainly focus on intracellular domain of molecule, perhaps due to localization of the great part of sortilin in cytoplasmic region 33, and develop monoclonal antibodies or inhibitory siRNAs against intracellular domain of this molecule, or amino acids and peptides which provide specific binding to surface sortilin molecule, Rabbani et al generated a mAb targeting surface expressed sortilin 34. Consequently, sortilin antibody could bind to extracellular epitope of sortilin protein in a number of ovarian carcinoma cell lines 34. Also, in another study carried out by Rabbani et al, this mAb could detect the surface sortilin in malignant B cells unlike previous studies and no study showed the surface expression of sortilin in normal or malignant B cells 35. The observed differences originate from the unique design of 2D8-E3 mAb that targets the N-terminal part of sortilin 34.
Furthermore, this report was the first that demon- strates in vitro invasive breast cell growth was blocked by the 2D8-E3 mAb, which detects the cell surface expressed sortilin. In a previous study on CLL cells using the same antibody, similar results were found in case of apoptosis induction 36. However, the significant role of sortilin in apoptosis induction has been shown through p75NTR-sortilin complex that binds to pro-BDNF in a high affinity manner in CRC cell lines 20. The exact mechanism of 2D8 mAb action is not clear; however, it was proved that sortilin in complex with Neurotensin receptor 1 (NT-R1) induces growth signaling pathways through neurotensin internalization 25. Hence, one possible explanation of 2D8 mAb-induced direct cell destruction would be its preventive effect on heterodimerization of sortilin with NTR1. Undoubtedly, further studies should be done in order to prove this hypothesis. In addition, beyond this killing mechanism of action, mAbs have the capacity to directly eradicate cancer cells or boost the chemo-or radio-therapeutic effects on cancer cells by modulating anti-apoptotic pathways 37.
Recently, the increased level of sortilin has been shown in ovarian cancer and its silencing through siRNA led to inhibition of cell proliferation (40.1%) in Caov-4 cells 38. Similarly, sortilin silencing in CAL-62 thyroid cancer cell line hampered the activation of extracellular signal-regulated kinases (ERK) which subsequently resulted in cell survival inhibition and a decrease in migration of malignant cells 39.
Analysis of apoptosis assays indicates that in spite of the fact that sortilin shows higher expression in MDA-MB231 cells when compared to 4T1 cells (90.3 vs. 79.2), the rate of apoptosis is significantly lower in human breast cancer cell line. This finding highlights the importance of arbitrary value in apoptosis induction. Arbitrary value represents the number of receptors on the tumor cells 40 and it is calculated by multiplication of MFI by the percentage. As shown in table 1, the arbitrary value for 4T1 cells is higher hence the treatment of two breast cell lines with equal amount of 2D8-E3 antibody resulted in stronger apoptosis in 4T1 cancer cells. For both cell lines, the viability of the cells was reduced to 19.2% for 4T1 cells and 23.7% for MDA-MB231 cells in 48 hr after induction. To the best of our knowledge, this study was the first that revealed the functional significance of surface sortilin in breast cancer and can be considered as a stepping stone for further studies for implication of this mAb in targeted cancer therapies.
Overall, in this study, an attempt was made to evaluate whether increased expression of surface sortilin in breast cancer cell line can be observed. Roselli et al 19 provide empirical support to the increased level of sortilin in breast cancer cell line as well as malignant tissues as compared to normal tissues. A surface protein, sortilin, is overexpressed in 4T1 and MDA-MB231 breast cancer cell lines and mAb against sortilin inhibits breast cancer cell growth in vitro and induces apoptosis in these cells. Although the inhibition of cell growth is often important in vitro, the effector functions of mAbs can also be significant in vivo. This highlights the significance of further investigation on the utility of this antibody in animal experiments, that will provide more evidence for sortilin as a promising agent for the treatment of tumors.
Conclusion :
This study indicates that sortilin expressed on the surface of breast cancer cells at high levels has a crucial role in survival of these cells. As mentioned above, the anti-sortilin mAb could induce apoptosis in both 4T1 8.45% (24 hr), 73.3% (48 hr), and 25.6% (72 hr) and MDA-MB231 12.3% (24 hr), 20.3% (48 hr), and 5.06% (72 hr) cell lines; hence, it can be served as a promising target among anti-cancer therapeutic agents.
It seems that further studies including other carcinoma cell lines and in vivo experiments are required to apply anti-sortilin mAb as a single therapeutic target not only in breast cancer but also in other malignancies as well.
Acknowledgement :
This work was supported by grants from Avicenna Research Institute, ACECR, Tehran, Iran (Grant No. 960205-012) and Tehran University of Medical Sciences, Tehran, Iran (Grant No. 97-03-87-40431).
Conflict of Interest :
The authors declare that they have no conflict of interests.
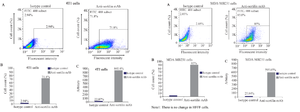
Figure 1. Reactivity of anti-sortilin monoclonal antibody clone 2D8-E3 to breast cancer and normal cell lines using flow cytometry. Left panel: A) 2D8-E3 could react with sortilin in 71.8% of 4T1 and 85% of MDA-MB231 cells, compared to HFFF cell (2.1%) as a normal sample. The values for isotype controls in all three cell lines have also illustrated. Middle panel: B) The same results illustrated as bars for better visualization. Right panel: C) The average FITC intensities were calculated through multiplying the mean fluorescence intensity by percentage of positivity (MFI×POP).
|
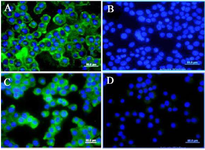
Figure 2. Immunocytochemistry (ICC) assay on breast carcinoma cell lines. Mouse monoclonal anti-sortilin antibody 2D8-E3 was used as a primary antibody and FITC-conjugated sheep anti-mouse antibody as secondary antibody (Green). DAPI was used for counterstaining the nucleus (Blue). A) (4T1 cells), C (MDA-MB231 cells), mouse IgG isotype controls. B) (4T1 cells) and D (MDA-MB231 cells).
|

Figure 3. A flow cytometric apoptosis assay was performed by anti-sortilin monoclonal antibody (2D8-E3) on breast cancer cell lines for 24, 48 and 72 hr. A) and B) The antibody could induce apoptosis (Early and late apoptosis) in 4T1 and MDA-MB231 cells after 48 hr. For better visualization of results a bar graph was drawn. The percentage of viable cells were 73.4 (24 hr), 19.2 (48 hr) and 45.5 (72 hr) for 4T1 cells and 78 (24 hr), 23.7 (48 hr) and 85.5 (72 hr) for MDA-MB231.
The viability of cells remains almost unchanged in both lines after 24, 48 and 72 hr of induction in HFFF cells as a normal cell line (C).
|

|

|
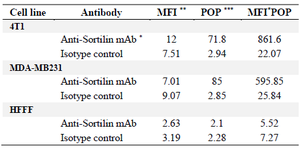
Table 1. Flow cytometry on breast cancer and normal cell lines
* Monoclonal antibody, ** Mean fluorescence intensity, *** Percentage of positive cells.
|
|