The Effect of Media Supplementation with Angiotensin on Developmental Competence of Ovine Embryos Derived from Vitrified-warmed Oocytes
-
Naderi, Mohammad Mehdi
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Borjian Boroujeni, Sara
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Sarvari, Ali
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Heidari, Banafsheh
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Akhondi, Mohammad Mehdi
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Zarnani, Amir-Hassan
-
Reproductive Immunology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 913 1821238, E-mail: shiraziabbas@yahoo.com, a.shirazi@avicenna.ac.ir
Shirazi, Abolfazl
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 913 1821238, E-mail: shiraziabbas@yahoo.com, a.shirazi@avicenna.ac.ir
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran
Abstract: Background: This study was aimed to assess the effects of angiotensin II (Ang II) supplementation to the In Vitro Maturation (IVM) and In Vitro Culture (IVC) media of vitrified-warmed ovine oocytes on their developmental competence and expression of Na+/K+/ATPase in resulting embryos.
Methods: The slaughterhouse-derived immature oocytes (n=1069) were randomly distributed into four experimental groups: groups I and II) IVM/IVF and IVC of fresh and vitrified oocytes without angiotensin supplementation (Control-Fresh and Control-Vit groups, respectively); group III) IVM of vitrified oocytes in the presence of Ang II followed by IVF/IVC (Vit-IVM group); and group IV) IVM/IVF of vitrified oocytes followed by IVC wherein the embryos were exposed to Ang II on day 4 of IVC (Vit-D4 group). The embryos were immunostained with primary antibodies against Na+/K+/ATPase α1 and β1 subunits.
Results: In Vit-IVM and Vit-D4 groups, the rates of expanded and total blastocysts on day 7 as well as the proportion of blastocysts on day 8 were increased. The expression of Na+/K+/ATPase α1 and β1 subunits were positively influenced by the addition of Ang II on day 4 (Vit-D4 group).
Conclusion: The addition of Ang II to the IVM and IVC media could improve blastocysts formation in vitrified sheep oocytes. This improvement might be related to the greater expression of Na+/K+/ATPase α1 and β1 subunits when Ang II was added during IVC.
Introduction :
Oocyte cryopreservation is becoming an integral part of Assisted Reproductive Technologies (ARTs) as an approach for female fertility preservation and conservation of endangered animal species as well as preserving the female genome of valuable laboratory models 1. For ovine oocytes, further attention to the effects of vitrification on gene and protein expression could be fruitful for overcoming the developmental blocks seen in this and other species 2. There are evidences indicating that alteration of culture conditions and media during oocyte maturation, fertilization, and embryo development have improved the production of embryos derived from vitrified oocytes 3-5. However, the rates of embryo development and pregnancy following cryopreservation of in vitro matured oocytes are lower than those obtained from in vivo matured oocytes 6-8. The presence of renin-angiotensin components has been shown in the oviduct, placenta, and foetal membranes of various species 9,10. The presence of high concentration of Ang II receptors, especially AGTR2, has also been reported in mouse, rat, and human fetuses 11. The high concentrations of Ang II in mammalian ovaries may indicate its role in ovarian function 12. In this context, the concentrations of Ang II has been increased in human and bovine follicular fluid at the time of ovulation and following exposure to Luteinizing Hormone (LH) or human Chorionic Gonadotropin (hCG) 6. Addition of saralasin (Ang II antagonist), however, could inhibit hCG-induced oocyte maturation 3. There is also evidence indicating direct effect of Ang II on ovarian steroidogenesis, follicular atresia, oocyte maturation, and ovulation 13.
Some genes were indicated as being controlled by Ang II such as Na+/K+/ATPase 14, Nicotinamide Adenine Dinucleotide Phosphate (NADPH), Mitogen-Activated Protein Kinases (MAPKs) and Cycloxygenase-2 (COX-2) 15. Studies have been indicated that sheep oocyte cryopreservation reduces mRNA levels of several critical genes including Na+/K+/ATPase 3,16.
There are some evidences indicating positive role of Na+/K+/ATPase during blastocyst formation in mouse including: (a) polarized dissemination of Na+/K+/ATPase just before beginning of cavitation, (b) increased Na+/K+/ATPase subunits expression during morula to blastocyst conversion, (c) inhibitory effect of ouabain (as a Na+/K+/ATPase inhibitor) on blastocyst development in some mammalians, and (d) positive role of Na+/K+/ATPase in function of trophectoderm tight junctions 17.
Considering the increase in Na+/K+/ATPase expression during morula to blastocyst transition 17 and the reduction of Na+/K+/ATPase mRNA abundance caused by vitrification 16, this study was aimed to evaluate the effect of addition of Ang II to the In Vitro Maturation (IVM) and In Vitro Culture (IVC) media of vitrified-warmed ovine oocyte on its subsequent developmental competence as well as the expression of Na+/K+/ATPase in resulting embryo.
Materials and Methods :
All experimental methods were confirmed by Avicenna Research Institute Bioethics Committee. Except where otherwise indicated, all chemicals were obtained from Sigma-Aldrich Co. (St. Louis, MO, USA).
Experimental groups: The immature oocytes, after aspiration, were randomly allocated into the following groups: groups I and II) IVM/IVF and IVC of vitrified and fresh oocytes without angiotensin supplementation (Control-Vit and Control-Fresh groups, respectively); group III) IVM of vitrified oocytes in the presence of 1010 M Ang II followed by in vitro fertilization (IVF)/IVC (Vit-IVM group); group IV) IVM/IVF of vitrified oocytes followed by IVC wherein the embryos were exposed to 1010 M Ang II on day 4 of IVC (Vit-D4 group).
The zygotes were then cultured in SOF medium at 39oC under condition of 7% O2, 5% CO2 and 88% N2 in humidified air for 8 days. The rates of cleavage and blastocyst/hatching were recorded on days 3 and 6 to 8, respectively (day 0 was defined as the day of fertilization). Each treatment was consisted of at least four replicates. To evaluate the effects of Ang II on Na+/K+/ATPase subunits expression, the morula and blastocyst embryos on day 8 were immunostained with different specific primary and a common Fluorescein Isothiocyanate (FITC)-conjugated secondary antibodies. The mean fluorescence intensity of the subunits was measured with ImageJ 1.37v software (National Institutes of Health, Bethesda, MD, USA).
In vitro maturation: Slaughterhouse-derived sheep ovaries were collected in 0.9% saline solution fortified with antibiotics at 35C and carried within 2 hr to the laboratory. All follicles measuring 2 to 6 mm were aspirated with a 20-gauge needle attached to a 5 ml syringe. Oocytes with at least three intact layers of cumulus cells and homogenous cytoplasm were selected for in vitro maturation. Oocytes were matured in medium consisted of Tissue Culture Media 199 (TCM199) supplemented with 10% (v/v) Fetal Bovine Serum (FBS), 0.2 mM Na-pyrovate, 0.05 U/ml follicle stimulating hormone (FSH), 100 IU/ml penicillin, and 0.1 mM streptomycin. The selected Cumulus Oocyte Complexes (COCs) were placed in maturation medium (10 oocytes/ 50 µl) and overlaid with mineral oil and were then incubated in a humidified atmosphere of 5% CO2 at 39oC for 24 hr.
Vitrification and warming: Vitrification was done based on Minimum Essential Volume (MEV) method using cryotop as cryodevice 16. Briefly, groups of five immature COCs were initially introduced into holding medium (HM) consisting of 20 mM HEPES-TCM supplemented with 20% (v/v) FBS for 1 min. The COCs were subsequently transferred into Vitrification Solution 1 (VS1) consisting of HM supplemented with10% (v/v) Ethylene Glycol (EG) and 10% (v/v) dimethyl sulfoxide (DMSO) for 30 s and were then incubated in vitrification solution 2 (VS2) consisting of HM supplemented with 20% (v/v) EG, 20% (v/v) DMSO and 0.5 M sucrose for 20 s. The COCs were then loaded on cryotop (Kitazato Ltd., Tokyo, Japan) with the least medium (<0.1 µl) and immediately plunged into liquid nitrogen. For warming, vitrified COCs were exposed to warming solution containing 1.25 M sucrose in HM for 1 min, followed by a second and third dilution in warming solutions containing 0.62 M and 0.31 M sucrose for 30 s of each.
In vitro fertilization: In vitro-matured COCs (n=1069) were fertilized with sheep sperm from a freezing batch of Shaal breed ram that had previously been successfully used in our laboratory. Semen was thawed at 36°C for 1 min, and was fractionated on discontinuous Percoll (Amersham Biosciences AB, Uppsala, Sweden) gradients as previously described 6. Sperm then were washed in HSOF [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)-synthetic oviductal fluid] by centrifugation at 750xg for 10 min at room temperature and were diluted in IVF medium with final concentration of 1.0×106 sperm/ml. Fertilization medium was Synthetic Oviductal Fluid (SOF), as previously indicated by Shirazi et al 18, supplemented with 20% (v/v) heat inactivated estrous sheep serum and 1% (w/v) heparin. Sperm suspension was added into the fertilization droplets containing 10 oocytes. Fertilization was done by co-incubation of sperm and oocytes in an atmosphere of 5% CO2 in humidified air at 39oC for 18 hr.
In vitro culture: After IVF, cumulus cells were removed by pipetting and the presumed denuded zygotes were placed in 20 µl culture drops (4-5 embryos/20 µl IVC medium) containing SOF supplemented with 1% (v/v) MEM-non essential amino acids, 2% (v/v) BME- essential amino acids, 8 mg/ml fatty acid free Bovine Serum Albumin (BSA) and 1 mM glutamine and 10-10 M Ang II on day 4 of IVC in Vit-D4 and D4 groups. Charcoal stripped FBS 10% (v/v) was added to the medium on the third and fifth day of culture. The osmolarity was maintained at 270 to 285 mOsmol. They were then cultured at 39oC under conditions of 7% O2, 5% CO2 and 88% N2 in humidified air. The percentage of cleaved embryos on day 3 and the percentage of blastocysts on days 6 to 8 were calculated on the basis of the number of oocytes at the onset of culture, and the percentage of hatched blastocysts on days 7 and 8 was expressed based on the number of blastocysts at the same day.
Immunostaining and quantification of fluorescence intensity: The primary antibodies against Na+/K+/ATPase subunits (Na+/K+/ATPase anti-α1 Monoclonal Antibody, Thermo scientific, Rockford, USA and Na+/K+/ATPase anti-β1 Monoclonal Antibody, Abcam, Cambridge) were diluted at 1:50 (v/v) and 1:100 (v/v), respectively. The secondary antibody (sheep anti-mouse antibody conjugated with fluorescin isothiocyanate; Avicenna Research Institute, Tehran, Iran) was diluted at 1:50 (v/v) with antibody diluents. For immunostaining, first the embryos at morula and blastocyst stages were fixed and prepared as previously described 19 and then exposed to primary antibody solution at 37°C for 4 hr followed by transfer to the secondary antibody solution at 37°C for further 4 hr. In negative controls, the primary antibodies were omitted. After immunostaining, the embryos were directly mounted on a glass slide and examined under a Nikon TE 300 (Nikon Corporation, Melville, USA) epifluorescence microscope with excitation wavelengths of 488 nm (for FITC). All images were prepared with a high-resolution Charge-Coupled Device (CCD) camera (Nikon Corporation) and duration of exposure for acquiring each type of fluorescence was kept constant. After subtracting the background, mean fluorescence intensity was measured by manually outlining all embryos with ImageJ 1.37v software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis: The differences among groups were analyzed by one-way ANOVA followed by post hoc Fisher LSD test using SigmaPlot (version 11.0). When equal variance test was failed, the treatments were compared by Student–Newman–Keuls method. When normality test was failed, the Kruskal–Wallis on ANOVA ranks was applied. Differences were considered significant when p<0.05. Data were expressed as mean±SEM. Data were collected over at least four replicates.
Results :
Effect of Ang II supplementation on embryo development: Addition of 10-10 M Ang II to IVM or IVC media in vitrified oocytes (IVM and D4 groups) significantly increased the rates of expanded and total blastocyst on day 7 (p=0.001 and p=0.02, respectively) as well as the greater proportion of total blastocyst on day 8 of culture (p<0.001) compared with control (Control-Vit group; Table 1). There was no significant difference in cleavage and expanded blastocyst rates on day 6 and hatched blastocyst rate on day 8 in vitrified groups with or without Ang II (Table 1).
Effect of Ang II supplementation on Na+/K+/ATPase subunits expression in embryos: There was a significant difference in Na+/K+/ATP ase subunit expression among treatment groups (Figure 1). The expression of Na+/K+/ATPase α1 and β1 subunits when IVC medium was supplemented with Ang II (Figure 2; p=0.003 and p<0.001, respectively) significantly increased compared to the control. Media supplementation with Ang II during IVM, however, had no influence on expression of Na+/K+/ATPase α1 and β1 subunits.
Discussion :
According to the increased concentration of Ang II at the time of ovulation in follicular fluid 6, and considering the up regulation of Na+/K+/ATPase at the time of embryonic genome activation, 8 to 16-cell stage 20,21, it was hypothesized that the addition of Ang II to the IVM and IVC media may play a significant role in sheep embryo development.
It has also been demonstrated that Na+/K+/ATPase expression is increased during morula to blastocyst transition 17,22,23. In our experimental condition, despite the general knowledge indicating the reductive role of cryopreservation on mRNA abundance, the expression of Na+/K+/ATPase in embryos derived from vitrified oocytes (Control-Vit group) was comparable to the embryos derived from fresh oocytes (Control-Fresh group). Nonetheless, the addition of Ang II to the IVC medium could increase Na+/K+/ATPase expression compared to other groups (Figure 1).
There are some evidences indicating the persuasive effect of Ang II on expression of Na+/K+/ATPase α1 and β1 subunits in various cell types 14,24-26. It has also been shown that Na+/K+/ATPase has a fundamental role in regulating mouse trophectoderm tight junction formation and function 22. Inhibition of Na+/K+/ATPase by ouabain was associated with failure of constitution of an efficient trophectoderm tight junctional seal that is required for expansion of the blastocyst cavity and hatching 17. On the other hand, blastocyst expansion only occurs when the tight junction permeability seal fully forms, to restrict the leakage of fluid via paracellular routes, ensuring the expansion of the cavity as fluid accumulates 20.
As shown (Table 1), among vitrified oocytes, the proportions of blastocysts on days 6 to 8 as well as the proportion of expanded blastocysts on day 7 of IVC in both Vit- IVM and Vit-D4 groups were higher than the corresponding value in Control-Vit (Table 1).
Therefore, it could be hypothesized that in vitrified groups, the increased proportions of expanded and total blastocysts might be related to the inductive role of Ang II in Na+/K+/ATPase expression which in turn could regulate the function of trophectoderm tight junction. Furthermore, the presence of Ang II in human follicular fluid and localization of its receptors on COCs 12, once more may suggest its positive role in oocyte competence which in our study was reflected in terms of greater development of subsequent embryo in treatment groups compared to the control.
In our experimental condition, the positive effect of AngII on expression of Na+/K+/ATPase α1 and β1 subunits in vitrified oocytes was only evident when Ang II was added during IVC (Figure 2). One possibility for the lack of positive effect of Ang II supplementation during IVM on Na+/K+/ATPase subunits expression might be related to the time interval between Ang II supplementation (during IVM) and assessment of Na+/K+/ATPase α1 and β1 expression (blastocyst stage on day 8). An increase in Na+/K+/ATPase genes expression during morula to blastocyst transition 22 may bring up the point that in our study condition the addition of Ang II on day 4 of IVC has a greater chance to affect Na+/K+/ATPase subunits expression in resulting morula and blastocysts compared to earlier stage supplementation (during IVM; Figure 2).
As demonstrated, Na+/K+/ATPase β1 subunit protein is required for blastocyst formation 17,21. In our study, the overall expression of Na+/K+/ATPase β1 subunit protein was greater than α1 subunit (Figure 2). Based on the documents, it seems Na+/K+/ATPase β1 subunit oversees the proper localization of Na+/K+/ATPase α1 subunit to the cortical membrane regions of each blastomere and also induces the proper distribution and assembly of tight junction-associated polypeptides (ZO-1 and occludin) to the apical membrane regions between differentiating trophectoderm cells. In addition, it has been indicated that the Na+/K+/ATPase β1 subunit is required to support early development to the morula (16–32 cells) stage of mouse embryos 17.
Other studies have demonstrated that Na+/K+/ATPase β1 subunit gene products display a dramatic up-regulation just before blastocyst formation suggesting up-regulation of this gene is required for cavitation and hatching process to occur. Based on what stated, it could be inferred that the up-regulation of Na+/K+/ATPase β1 subunit should have a greater positive impact on blastocyst and hatched blastocyst formation in Ang II treated groups compared to α1 subunit (Table 1).
Concerning the Na+/K+/ATPase α1 subunit, as documented, its mRNAs are present throughout preimplantation development and display a much more gradual increase as the embryo progresses to the blastocyst stage 17,21. In our study, despite the lower expression of α1 compared to β1 subunit, it seems it may have a role on blastocyst formation in embryos exposed to the Ang II during IVC.
Now the question arises that if the higher developmental competence of vitrified oocytes is related to the higher expression of Na+/K+/ATPase α1 and β1 subunits, what the reason for the higher blastocyst rate is in Vit-IVM compared to Control-Vit group. Therefore, apart from the positive effect of Ang II on Na+/K+/ATPase α1 and β1 expression, there must be another reason for the positive effect of AngII on blastocyst rate in Vit-IVM group which needs to be further investigated.
Conclusion :
The addition of Ang II to the IVM and/or day 4 of IVC media significantly increased the rate of blastocyst in vitrified oocytes. The higher expression of Na+/K+/ATPase α1 and β1 subunits in embryos derived from vitrified oocytes might be the reason for the higher blastocyts rate in vitrified oocytes subjected to AngII during D4 of IVC.
Acknowledgement :
The authors would like to thank Avicenna Research Institute (grant no. 90-32) for financial support and providing the opportunity to conduct the study.
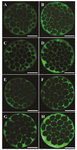
Figure 1. Immiunostaining of Na+/K+/ATPase α1 and β1 subunits in embryos derived from fresh and vitrified oocytes. Green color in each micrograph indicates the presence of α1 and β1 Na+/K+/ATPase subunits probed by the respective primary antibodies which has been subsequently identified by secondary antibody conjugated with fluorescin isothiocyanate. The intensity of Na+/K+/ATPase α1 and β1 subunits in embryos derived from Control-fresh (A and B, respectively) and Control-Vit (C and D, respectively) groups. The intensity of Na+/K+/ATPase α1 and β1 subunits in embryos derived from vitrified oocytes supplemented with AngII during IVM (E and F, respectively). Expression of Na+/K+/ATPase α1 and β1 subunits in embryos derived from vitrified oocytes supplemented with Ang II during Day 4 of IVC (G and H, respectivly). Bars, 40 µm.
|
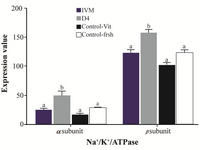
Figure 2. Effect of Ang II supplementation in culture medium during IVM and IVC (day 4) on expression of α1 and β1 Na+/K+/ATPase subunits in embryos derived from vitrified and fresh oocytes. Mean fluorescence intensity was measured by manually outlining the embryos with ImageJ 1.37 v software. The values are expressed as mean±SEM. For each Na+/K+/ATPase subunit, bars with different lowercase letters indicate significant difference between experimental groups ( IVM, D4, and control; p<0.05).
|
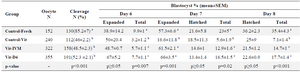
Table 1. The presence of angiotensin in culture media during IVM or Day 4 of IVC on embryo development
a, b) Numbers with different lowercase superscript letters in the same column differ significantly.
The percentage of blastocysts at days 6 to 8 were expressed based on oocytes number at the onset of culture, and the percentages of expanded and hatched blastocysts at days 7 and 8 were expressed based on the total number of blastocysts at the same day.
|
|