Cytogenetic Alterations in Preimplantation Mice Embryos Following Male Mouse Gonadal Gamma-irradiation: Comparison of Two Methods for Reproductive Toxicity Screening
-
Salimi, Mahdieh
-
Department of Medical Genetics, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
-
 Mozdarani, Hossein
Department of Medical Genetics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, Tel: +98 21 82883830; E-mail:mozdarah@modares.ac.ir
Mozdarani, Hossein
Department of Medical Genetics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, Tel: +98 21 82883830; E-mail:mozdarah@modares.ac.ir
-
Department of Medical Genetics, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
Nazari, Elmina
-
Department of Science and Research, Azad University and Royan Institute, Tehran, Iran
Abstract: Background: Genome instability is a main cause of chromosomal alterations in both somatic and germ cells when exposed to environmental, physical and chemical genotoxicants. Germ cells especially spermatozoa are more vulnerable to suffering from DNA damaging agents during spermatogenesis and also more potent in transmitting genome instability to next generation.
Methods: To investigate the effects of γ-rays on inducing abnormalities manifested as numerical Chromosome Aberrations (CA) and Micronucleus (MN) in preimplantation embryos, adult male NMRI mice were irradiated with 4 Gy of γ-rays. They were then mated at weekly intervals with superovulated, non-irradiated female mice in 6 successive weeks. About 68 hr post coitous, four to eight cell embryos were retrieved and fixed on slides using standard methods in order to screen for CA and MN.
Results: In embryos generated from irradiated mice, the frequency of aneuploidy and MN increased dramatically at all post-irradiation sampling times as compared to the control (p<0.01). The frequency of embryos expressed MN was much higher than chromosomally abnormal embryos, although the trend of MN formation was similar to chromosomal abnormalities seen in corresponding sampling times.
Conclusion: Irradiation of sperms at any stages of spermatogenesis may lead to stable chromosomal abnormalities affecting pairing and disjunction of chromosomes in successive preimplantation embryos that are expressed as MN. Although chromosome analysis of embryos showed various types of chromosomal abnormalities, MN assay provide a simpler and faster technique for investigating the genotoxicity of agents affecting embryos at preimplantation stages.
Introduction :
Spermatogenesis is a long, complex and finely tuned process 1. During this process, the developing germ cells are sensitive to endogenous and exogenous stress. Cancer therapies such as radiotherapy and chemotherapy can cause temporary or permanent impairment of fertility in male cancer patients who usually are in the reproductive age 2-4. Therefore, an important goal of successful treatment is to minimize the cytotoxic impact of the treatment in order to maximize chances of re-initiating spermatogenesis while still efficiently killing cancerous cells. To this end, it is necessary to understand how radiation affects the differentiating germ cells and thus produces infertility in male mammals or abnormality in subsequent embryos or fetuses.
Sperm DNA damage is gaining interest as a potential cause of infertility, and it may be initiated by a wide range of causes such as drugs, chemotherapy, radiation therapy, cigarette smoking and environmental toxins, genital tract inflammation, testicular hyperthermia, varicoceles, hormonal factors, etc. 5. The normality of sperm nuclear DNA plays a critical role in mammalian fertilization and subsequent embryonic development 6.
Germ cell mutagens such as ionizing radiation may lead to induction of an elevated germ line mutation rate in the directly exposed parents. These mutational events may have an indirect effect on genome stability which is transmitted through the germ line of irradiated parents to their offspring 7,8. Male and female germ cells vary in their sensitivity to the mutagenic effects of chemotherapy and radiotherapy, depending on their stage of maturation and the agent used 9,10.
The effects of radiation on human beings include miscar¬riage, stillbirth, and malformation due to a genetic disorder in the paternal germ cell, as well as an increased incidence of cancer 7,11,12. In particular, the incidence of genetic disor¬ders in the descendent generation is likely to result from genome instabilities in the parent’s generation 13.
It has been demonstrated that sperm cells with damaged or fragmented DNA can fertilize oocytes in vitro 14. Some authors consider that this also happens in vivo 15 and that highly motile mouse sperm did not differ in types and frequencies of chromosomal abnormalities from those not selected for motility 16.
It has been shown that DNA-damaged sperms have the ability to fertilize the oocyte but that embryonic development is very much related to the degree of DNA damage. The majority of de novo structural chromosome aberrations in fetuses and newborns are considered to be of paternal origin, especially of sperm origin 17.
Due to the importance of the paternal germ cell in genetic disorders caused by radiation, apoptosis, gene mutation, repair capa¬bilities, and chromosome aberrations of spermatogonia were used as endpoint markers for evaluation 18-23. Investigation of DNA damage and chromosomal abnormality due to radiation in germ cells of male and female and their embryos can be carried out by several methods. One simple and economical method for this is the micronucleus test in interphase cells 24.
Micronuclei reflect structural and/or numerical chromosome aberrations arising during mitosis 25-28. The quantification of MN is simple and fast, and it does not require the presence of cells at metaphase stage 27. Some studies have used MN assay to investigate the irradiation effects on chromosomal aberrations and genome instability 29.
The aim of this study was, therefore, to investigate the effects of paternal gamma-irradiation of mice at various time intervals on the frequency of micronuclei and numerical chromosome aberrations and compare the efficacy of these two methods for screening transgenerational genome instability induced by physical and chemical agents.
Materials and Methods :
Animals and test groups: Six to eight week old NMRI mice with a mean weight of 30±5 gr were purchased from Razi Institute (Karaj, Iran). Male mice were housed singly in plastic cages; females were housed in a group of 4-5/cage at least for one week before being used for experiments. The animals were housed in a room kept in mesh cages at 22ºC with a cycle of 10 hr darkness and 14 hr light and 60-70% relative humidity. Mice were fed with standard breeding granulated diet and water ad libitum. Females and males were randomly assigned to control or test groups and mated overnight after induction of superovulation in females using intraperitoneal (i.p) injection of 10 International Units (IU) of pregnant mare’s serum gonadotrophin folligon (PMSG; Intervet, Germany) followed by injection of 10 IU of Human Chorionic Gonadotrophin (HCG; Organon, UK) with a 42-48 hr interval. Four days after gamma-irradiation, irradiated male mice were mated with superovoulated females in 6 successive weeks at weekly intervals.
This study was approved by the Ethics Committee of Tarbiat Modares University and animals were treated according to the university regulations.
Gamma-irradiation and coupling: Mice were whole body irradiated with 4 Gy gamma rays generated from a cobalt-60 source (Theratron II, 780 C, Canada) at a dose rate of 132 cGy/min, with source to sample distance of (SSD)=82 cm, field size of 20×20 cm at room temprature (23±2ºC). Four days after gamma-irradiation, irradiated male mice were mated with superovoulated un-irradiated females in 6 successive weeks at weekly intervals. Three to five irradiated male mice were assigned for coupling in each experimental group. Two un-irradiated superovoulated female mice were transferred with an irradiated male in a cage for an overnight to mate. The next morning female mice were checked for Vaginal Plaugh (VP). A VP positive female was considered as a pregnant mouse. A control group consisted of un-irradiated animals was assigned for each experimental group. All experiments were repeated three times.
It is worthy to mention that several authors used the dose of 4 Gy for investigating radiation induced genomic instability 30, in vitro cytogenetic effects on human and mouse germ cells 31,32 and prenatal effects of gamma-irradiation 33.
Embryo recovery: About 68 hr post coitous (p.c), the pregnant females were sacrificed by cervical dislocation method and their oviducts were flushed using a special flush syringe (Supa, Iran) filled with 37ºC incubated T6 medium [ingredients for pH of 7.2-7.4; NaCl (4.73 mg/ml), KCl (110 µg/ml), NaH2PO4 (50 µg/ml), MgCl2.6H2O (100 µg/ml), CaCl2.2H2O (260 µg/ml), NaHCO3 (2.10 mg/ml), phenol red (10 µg/ml), ethylenediaminetetraacetic acid (EDTA, 6 µg/ml), glucose (1 mg/ml) and Na-pyruvate (30 µg/ml) purchased from Sigma, St. Louis, MO, USA; penicillin G (60 µg/ml) and streptomycin (50 µg/ml) from Seromed, Berlin, Germany and Na-lactate (1.98 µg/ml) from Merck, Darmstadt, Germany]. The flushing was done under a stereomicroscope (Hund-Wetzlar, Wetzlar, Germany) to obtain 4-8 cell embryos. The collected morphologically normal embryos were transferred to fresh T6 medium supplemented with 15 mg/ml bovin serum albumin (BSA, Sigma) containing 0.2 µg/ml colcemid (Gibco BRL, Lifetech, USA) incubated in a humidified CO2 incubator (Lifetech, USA) at 37ºC for 16-20 hr (Colcemid was used only for metaphase analysis not for MN assay).
Slide prepareation for cytogenetic analysis: For cytogenetic analysis, Dyban method, which is a suitable method for analyzing chromosomes of embryo cells, was used with some modifications 34. Briefly, the zona pellucida was removed by the use of Tyrode's acid [ingredients for pH=2.5; NaCl (8 mg/ml), KCl (2 mg/ml), MgCl2.6H2O (0.1 mg/ml), CaCl2.2H2O (0.25 mg/ml), glucose (1 mg/ml) and polyvinylpyrrolidone (4 mg/ml) all from Sigma, St. Louis, MO, USA]. This process was followed under a stereomicroscope to avoid damage to the blastomers. Then embryos were transferred to a watch glass containing 1% sodium citrate (Sigma, St. Louis, MO, USA) as a hypotonic solution for 30 min. Embryos were placed on a pre-cleaned slide and fixed with a drop of fixative consisting of methanol and acetic acid (3:1) (Merck, Darmstadt, Germany). After leaving overnight at room temperature, slides were stained in 3% Giemsa (Merck, Darmstadt, Germany) for 3 min and cells were analyzed under a light microscope (Nikon, Kawasaki, Japan) at ×1000 magnification to screen micronuclei in blastomers and numerical chromosome abnormalities. Figures 1 and 2 show sample metaphase and blastomers with or without micronuclei after staining in Giemsa.
Statistical analysis: Data were statistically analyzed and the significance of any inter-group differences was evaluated with χ2 and Mann-Whitney U-test using SPSS (version 16) software. One way analysis of variance (ANOVA) was used to compare three or more groups. A p-value of less than 0.05 was considered significant.
Results :
Results are summarized in tables 1 and 2 and shown in figure 3. As seen, radiation dramatically increased the frequency of embryos with abnormal metaphases (Table 1) and the yield of MN in embryos generated from irradiated males was compared to embryos generated from non-irradiated male mice (Table 2). As seen in figure 3A, in the embryos generated from irradiated males from 1st to 6th weeks post-irradiation, 14.33, 17.7, 23.3, 29.7, 35 and 41% of embryos contained abnormal metaphase plates respectively and all values were significantly higher than the control group value of 8.7% (p<0.01). The frequencies of embryos containing MN were 19.9, 24, 36.8, 41.57, 53.9 and 66.7%, respectively which were all significantly higher than the control group value of 10.6% (p<0.01) (Figure 3A). Similar trend of MN formation following irradiation was observed when the frequencies of MN were analyzed per 100 cells instead of 100 embryos (Figure 3B). In table 2 and figure 3, the frequency of micronuclei is expressed as MN per cell because all the embryos did not contain similar number of cells. All the embryos retrieved were not at eight cell stage, i.e. different embryos contained different number of cells. This analysis might provide more accurate estimate of micronuclei formation in cells rather than assuming all embryos have similar number of cells.
Data shown in figure 3 clearly indicate that the frequency of micronucleated embryos and cells in all post-irradiation mating times was significantly higher than embryos or cells with abnormal metaphase plates (p<0.05 for weeks 1 and 2; p<0.01 for weeks 3-6). However, the increasing trend of abnormal metaphase and micronuclei formation was similar.
Discussion :
Studies of preimplantation stage embryos by classic cytogenetic techniques have limitations, starting with the need for good metaphase spreads when only one third of all analyzed embryos may show good quality metaphases (e.g., Table 1) 35,36. MN test is a reliable in vivo test for evaluation of the clastogenic effects of mutagens and radiation. MN arises from acentric chromosome fragments or chromosomes which are not incorporated into daughter nuclei during mitosis 25. MN scoring in interphase cells has been proposed and used as the quick and easy substitute for the more difficult and time consuming metaphase aberration analysis 37- 39.
Sperm DNA fragmentation affects sperm motility and fertilization rates 40. It has been reported in vivo that the likelihood of boar spermatozoa with unstable chromatin to reach and to fertilize the oocyte is very low 41. There is evidence suggesting that the journey of the sperm cells from the site of deposition to the site of fertilization is both dynamic (by the sperm and the female tract) and highly complex 42. Passage of sperm through female reproductive tract is regulated to maximize the chance of fertilization and to ensure that sperm cells with normal morphology and vigorous motility will be the ones to succeed 43.
It has been reported that in vitro, sperm with single stranded or denatured DNA bind less or do not bind at all to the Zona Pellucida (ZP) 18. In pigs, spermatozoa with stable chromatin are more likely both to bind to the oviduct and to traverse the reproductive tract in vivo 41, ultimately reaching the oocytes and penetrating the zona pellucida. Since the female reproductive tract does not assess the sperm DNA quality directly, the selection has to be based on sperm phenotype and function 44.
Other studies showed that radiation-induced DNA damages in spermatozoa may be transmitted to the next generation without being selected at fertilization, because it is previously shown that spermatozoa can retain a high fertilizing ability even after a high dose of irradiation 45. In an investigation on in vitro fertilization rate of mouse eggs with sperm after X-irradiation at various spermatogenesis stages, Mastuda et al 46 have shown that the number of fertilized eggs seemed to remain constant at control level until the 4th week after X-irradiation and then it reached to a minimum level in the 6th week. The response to radiation exposure is very much dependent on the developmental stage of germ cells during which this exposure takes place. These changes are explained in terms of the differential sensitivity of cells to killing and aberration induction in different phases of cell cycle. Results obtained in the present study, as shown in figures 3A and B, are in agreement with other previous reports indicating radiosensitivity of all cell lineage in the spermatogenesis process 19,32,45-50.
As the data indicates, the frequency of numerical chromosome abnormalities and MN presence in embryos generated from gamma-irradiated male mice for all six weeks post-irradiation is significantly higher than that of the control group (p<0.01). Moreover, the frequency of abnormalities sharply increased from the 4th through the 6th weeks post-irradiation.
MN is the result of chromosomal aberrations induced during preceding mitotic division of blastomers. These are from acentric fragment or lagging chromosomes induced by mutagens or clastogens such as ionizing radiation or are the result of non-disjunction 25,39,51. Irradiation of embryos in the first mitotic division could induce chromosomal abnormalities after several blastomers divisions in embryos. Recently, it was shown that irradiation of germ cells before mating leads to increased frequencies of chromosomal aberrations in subsequent pre-implantation embryos 52. Numerical abnormal metaphase plates may contain more than 1 chromosome abnormality that each one by itself can be expressed and visualized as MN in subsequent generating embryos. As indicated in figure 3, the increase of MN as compared with abnormal metaphase plates might be due to this reason.
Required time for spermatogenesis in mice for spermatozoa development from the stem cells is more or less constant (about 6 weeks). Accordingly, the fertilizing spermatozoa in the first week post gamma-irradiation has been in its spermatid stage at the time of irradiation, also gamma-irradiated early spermatid, secondary spermatocyte, early spermatocyte and spermatogonia stages act as a fertilizing spermatozoa in 2nd, 3rd, 4th, 5th and 6th weeks post-irradiation, respectively 53.
Data shown in tables 1 and 2 as well as figure 3 suggest that gamma-irradiation affects all the stages of spermatogenesis cycle in the male mice for inducing micronuclei and numerical chromosome aberrations. As seen, the increased frequencies of MN and numerical chromosome aberrations in male mice for all mating times post-irradiation were significantly different from the controls’ (p<0.01). There was a sharp increase in MN frequency and numerical chromosome aberrations from 4th to 6th weeks post-irradiation. These results suggest that gamma-irradiation affects all the stages of spermatogenesis cycle in the male mice, but spermatocyte and spermatogonia stages are the most radiosensitive stages for inducing numerical chromosome abnormalities. These abnormalities may be due to translocations induced in chromosomes that affect chromosome pairing and meiotic segregation in male mice resulting in aneuploid lagging chromosomes 52 which are expressed as MN.
Conclusion :
In conclusion, it was shown that first, the genome instability induced in male germ cells at any stages of spermatogenic cycle will be translated into the subsequent embryos formed by these germ cells as chromosomal abnormalities or micronuclei; i.e. transgenerational genome instability. Therefore, the benefit of this type of research is showing that the effect of DNA damaging agents such as ionizing radiation is not limited to the spermatogenesis process, rather they can be transmitted to the next generation. These types of damages are the main causes of embryonic death, implantation failure, and embryonic abnormalities in later stages. Second, micronuclei are usually considered as chromosome fragments of lagging chromosomes observable after first mitotic cycle. It was shown that DNA damage in any stage of spermatogenic cycle will lead to the formation of micronuclei in subsequent embryos. The results also indicate that micronuclei assay provide an easy and simple method for screening transgenerational genome instability in preimplantation embryos induced by chemical and physical agents as compared to more difficult and time consuming metaphase analysis techniques.
Acknowledgement :
Authors would like to thank Dr. M.H. Zahmatkesh for his help in gamma-irradiation of mice. This work was supported in part by the Royan Institute.
Conflict of Interest :
The authors declare no conflict of interests.
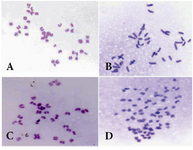
Figure 1. Metaphase plates prepared for blastomers showing A) normal; B) hyperdiploid; C) hypodiploid; D) near triploid metaphase spreads
|
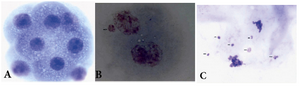
Figure 2. Photomicrographs of A) blastomers showing 8 cell normal embryo; B) two cell embryo having one micronucleus; C) two cells at anaphase with lagging chromosomes (arrows) eventualy forming micronuclei
|
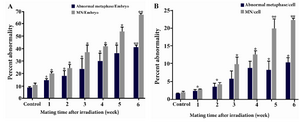
Figure 3. Percentage of chromosomal abnormalities and MN presence in A) 4-8 cell preimplantation embryos; and B) cells generated from gamma-irradiated (4 Gy) male. Whole body irradiated males were mated successively at weekly intervals from 1-6 weeks after irradiation. Error bars show standard error of mean values calculated from three independent experiments.
* denotes the p-value <0.01 and ** denotes p-value <0.001 as compared to control values.
|
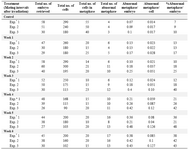
Table 1. Frequency of abnormal metaphases in analyzed embryos and cells after paternal irradiation (4 Gy gamma rays), before mating with non-irradiated female mice at weekly intervals. Results were obtained from three independent experiments. Data in each experiment represent pooled data obtained from a group of 4-5 VP positive (pregnant) mice
* Exp=Experiment
|
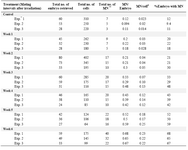
Table 2. Frequency of micronuclei in analyzed embryos and cells after paternal irradiation (4 Gy gamma rays), before mating with non-irradiated female mice at weekly intervals. Results were obtained from three independent experiments. Data in each experiment represent pooled data obtained from a group of 4-5 VP positive (pregnant) mice
* Exp=Experiment; ** MN=Micronucleus
# The column showing MN/cell is indicative of the frequency of MN observed in total cells of analyzed embryos. This was done because all the embryos did not contain similar number of cells
|
|