Genotyping of Polymorphic Microsatellite Markers Linked to RB1 Locus in Iranian Population
-
Mohamad Zahery, Saman
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture, and Research (ACECR), Tehran, Iran
-
Monoclonal Antibody Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
Saliminejad, Kioomars
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
-
 Ahani, Ali
Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, +98 21 22432020; a.ahani@avicenna.ac.ir
Ahani, Ali
Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran, +98 21 22432020; a.ahani@avicenna.ac.ir
-
Reproductive Biotechnology Research Center, Avicenna Research Institute, Academic Center for Education, Culture and Research (ACECR), Tehran, Iran
Abstract: Background: Retinoblastoma is the most common intraocular tumor in childhood and mutation in the RB1 gene will trigger the tumorigenesis. So far, a wide range of the mutations along the length of RB1 gene have been reported. However, some could not be detected by common detection methods. In such condition, linkage analysis using microsatellite markers is suggested to trace unknown RB1 mutations in the affected family. The aim of the present study was to evaluate the heterozygosity rates and genotyping of three microsatellite markers near or inside of the RB1 gene.
Methods: Totally, 120 unrelated healthy people from Fardis, Karaj, Iran were recruited and from each participant genomic DNA was extracted from 5 ml of peripheral blood. Three microsatellite markers D13S153, D13S156 and D13S128 located within or adjacent to the RB1 gene were selected for linkage analysis. The reliability of microsatellite markers and linkage analysis were investigated in 10 members of 2 families with familial retinoblastoma.
Results: Our results showed that heterozygosity rates for the three markers D13S153, D13S156 and D13S128 were 74, 70 and 78%, respectively. On the other hand, 2 and 36 out of 120 people were homozygote and heterozygous for all loci, respectively.
Conclusion: Given the heterozygosity rates, it may be concluded that all microsatellite markers D13S153, D13S156 and D13S128 are informative and have a high rate of heterozygosity and sensitivity. Therefore, tracing the unknown RB1 mutated alleles using linkage analysis in Iranian family with familial retinoblastoma could be recommended by means of these three microsatellite markers.
Introduction :
Retinoblastoma is the most common intraocular malignancy in children. It arises after two distinct inactivating mutational events in both alleles of the RB1 gene in an immature retinal cell. Roughly half of the patients with retinoblastoma inherit one mutated RB1 allele. Among them, many will be affected by bilateral retinoblastoma in childhood. Additionally, near one third of bilateral patients (15-20% totally) have a positive familial history for the retinoblastoma or retinoma. Detection of RB1 mutations in affected families will enhance the quality of clinical management of the probands and relatives at risk (1,2).
Genetic diagnosis has a great impact on management of families with retinoblastoma (3). In many developed countries, mutation detection as a routine part of clinical approaches is suggested to all affected families with retinoblastoma. Once the mutation was detected, to avoid transmission of the mutation, Pre-implantation Genetic Diagnosis (PGD) and Prenatal genetic Diagnosis (PND) as well as screening of carriers among proband’s sibs could be performed (3). Because all sibs of the proband are at risk of developing retinoblastoma, they periodically undergo a series of clinical examinations including Examination Under Anesthetic (EUA) to diagnose and treat tumors as early as possible. With carrier testing in the families it is no longer needed to EUA and fundoscopic examinations of the healthy sibs. In such a way, if the proband’s RB1 gene status is determined by genetic testing, only those relatives with the mutation require clinical surveillance, whereas those proven to be non carriers require no further examinations (1,2,4-7).
In the past two decades, wide spectrums of different mutations in the promoter, exonic and intronic regions of the RB1 gene, have been reported which are actually scattered along the RB1 gene. Unfortunately, there is no real hot spot in the RB1 gene and as a result many of these mutations are unique to a person and have not been seen in other patients. Due to low frequency of each unique mutations and distribution of other mutations all over the RB1 gene, identification of genetic causes of retinoblastoma is difficult and time consuming (8-22).
In some patients, in spite of family history of retinoblastoma no mutation is detected, so performing PGD and PND for patients with unknown mutation is impossible. To overcome this problem, it is usually suggested to use linkage analysis and define the likelihood of transmission of mutant allele to the proband with a high accuracy. For linkage analysis in affected families, easily detectable linked markers to the RB1 gene with high rates of heterozygosity must be available and evaluated allele’s frequency for each of the relevant markers in the population (23-28). However, to the best of our knowledge, allele frequencies for the microsatellite markers linked to the RB1 gene have not been evaluated and characterized among Iranian people. Therefore, the aim of the present study was to define the polymorphism and genotyping of the three microsatellite markers within or adjacent to the RB1 gene in Iranian population.
Materials and Methods :
Subjects: A total of 120 unrelated healthy people from Fardis, Karaj, Iran were recruited for this study during 2010-2011. All participants were ethnically Iranian with no family history of retinoblastoma. From each person 5 ml of peripheral blood in EDTA containing tubes were collected. Genomic DNA from peripheral blood was extracted by a standard salting out method as described elsewhere (29). Informed consent was obtained from each participant. The study protocol was approved by the Ethics Committee of the Avicenna Research Institute. The reliability of microsatellite markers and linkage analysis were investigated in 10 members of 2 families with familial retinoblastoma.
Polymerase chain reaction: Three microsatellite markers D13S153, D13S156 and D13S128 located within or adjacent to the RB1 gene on chromosome 13q were selected for linkage analysis. Primer sequences are shown in table 1. The PCR reactions carried out in final volume of 25 µl containing: 2.5 µl of 10× PCR buffer (Roche, Germany), 1.5 mM MgCl2 (Roche, Germany), 0.4 mM of each dNTP (Fermentas, Germany), 10 pmol of each primer, 50 ng template DNA, 1 U Taq DNA polymerase (Roche, Germany) and sterile distilled water up to 25 µl. PCR was performed with an initial denaturation step at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s, ended by a final extension at 72°C for 10 min. Then PCR products were separated on 12% polyacrylamide gels and visualized by Silver Nitrate staining.
Genotyping: After electrophoresis and analyzing the bands pattern for each sample, those which had the fragments with a higher likelihood of homozygosity were chosen and amplification by PCR was repeated for them. Then, the PCR products were separated on the 12% polyacrylamide gels and followed by Ethidium Bromide staining. In the next step to avoid contamination by the shadow and stutter bands, the main band in each lane were cut and transferred to 1.5 ml microtube. The pieces of gel containing the band were crushed and homogenized by pestle and then soaked in 100-500 µl PBS buffer (depending on the amount of the cut gel). Then, the dissolved DNA fragments were precipitated using sodium acetate and ethanol (30,31). The purified DNA was sequenced using Big Dye terminator chemistry and analyzed by Chromas software.
Results :
Three microsatellite markers D13S153, D13S156 and D13S128 located within or adjacent to the RB1 gene on chromosome 13q were checked using PCR in the 120 selected people. Analysis of microsatellites revealed that heterozygosity rates for the three markers D13S153, D13S156 and D13S128 were 74, 70 and 78%, respectively. Our results showed that 2 and 36 people were homozygote and heterozygote for all three loci, respectively. Three examples of PCR results for the microsatellites markers are shown in the figures 1and 2.
In order to define the number and the type of the repeats in each locus, the largest and the smallest bands as well as some bands in the middle range were extracted from the gel and sequenced. The numbers of alleles for each marker are summarized in table 2. As clearly depicted in figures 1 and 2, in the top of each band, stutter and shadow bands are located. These DNA fragments are produced during PCR by slipped strand mispairing mechanism and also were seen in the results of other studies (30,31).
Linkage analysis in 10 members of 2 families with familial retinoblastoma confirmed the reliability of microsatellite markers in tracing the responsible allele. The pedigree analysis for an engaged family clearly showed that these markers are linked to the mutant allele (Figure 3).
Discussion :
Over the last three decades, many of molecular techniques for detection of mutations have been developed. Therefore, potentially molecular causes of genetic diseases could be clearly detected. However, some of mutations in families affected by various genetic diseases such as retinoblastoma could not be detected by common approaches. In the absence of complement and alternative methods we are unable to assist families with familial retinoblastoma and unknown mutations. To overcome this problem, linkage analysis using polymorphic markers has been suggested as an alternative procedure (32-36). Linked markers are genetic polymorphisms which are easily detected. So far, many of these markers are identified including SNP (Single Nucleotide Polymorphism), RFLP (Restriction Fragment Length Polymorphism), VNTR (Variable Number of Tandem Repeats) and microsatellite markers. According to the facts, in the present survey three microsatellite markers D13S156, D13S153 and D13S128 were used to trace the unknown RB1 mutation in familial retinoblastoma.
The marker D13S153 (Rbi2) is inside the second intron of the RB1 gene. This marker has been investigated in many studies and its heterozygosity rate in many populations has been calculated (24,26,28,37,38). However, to the best of our knowledge, this is the first study which investigated the heterozygosity rate of D13S153, as well as number of alleles in an Iranian population.
Our results showed that 89 out of 120 people (~74%) were heterozygote for D13S153 marker. The heterozygosity rates for the two other markers D13S156 and D13S128 were 70 and 78%, respectively. The heterozygosity rates in the other studies are slightly higher than our results, for example in the study by Choy et al the heterozygosity rates for these markers were 64, 89 and 92% respectively (39).
These differences could be explained by various hypotheses. It may be due to genetic composition of the studied populations. High rates of inbreeding will decrease the frequency of heterozygote people. In the present study, samples were collected from a city (Fardis, Karaj, Iran) which has a high immigration rate, so it could be claimed that the studied population is a small sample of Iranian gene pool. However, we expected a higher heterozygosity rates in Iranian population.
According to our results, 2 and 36 of people were homozygous and heterozygous for all the three microsatellite markers, respectively. These results indicated that chosen microsatellite markers are informative and have a high sensitivity for tracing unknown RB1 mutation or responsible allele in affected family.
Linkage analysis despite its advantages in tracing unknown mutation has some limitations. Firstly, for linkage analysis in a family at least two affected individuals, if possible in two generation, must be available. Secondly, if the members of a family are homozygote for all three loci, linkage analysis using these markers is impossible. Thirdly, crossing over in the distance between the markers and the RB1 gene may occur. However, using an intragenic marker along with two markers flanking to RB1 gene will decrease the false results due to crossing over.
The polymorphic markers are also used to analyze Loss of Heterozygosity (LOH) of a specific locus. In retinoblastoma, inactivation of the both alleles of the RB1 gene in immature retinal cells led to tumorigenesis. According to the Knudson two-hit hypothesis, the first mutation is inherited via germline and occurrence of the second one in these cells trigger carcinogenesis. Based on previous studies (1,8,10,21), the most common cause of second allele mutation is LOH. In the gene testing procedure, when the tumor tissue as well as blood sample was available, checking the second mutation in the tumor tissue could be performed through LOH analysis.
Conclusion :
Finally, it could be concluded that the three microsatellite markers D13S153, D13S156 and D13S128 have high rates of heterozygo-sity and could be recommended for linkage analysis in Iranian family with familial retinoblastoma and unknown RB1 mutations.
Acknowledgement :
The authors would like to thank all participants for their collaboration and providing blood samples.
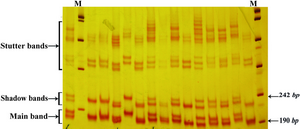
Figure 1. Band pattern of D13S153 marker on 12% poly-acrilamide gel. The first lane in the right side is marker VIII (Roche)
|
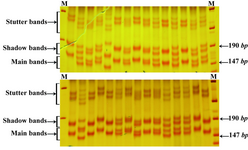
Figure 2. Band pattern of D13S128 marker on 12% poly-acrilamide gel. The first lane in the left side is marker VIII (Roche) and the first lane in the left side is 100 bp marker (Fermentase)
|
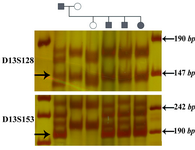
Figure 3. Linkage analysis in a family with retinoblastoma (Patient no. 200 is proband). The arrows show the markers which are linked to the mutant allele. The two microsatellite markers, D13S128 and D13S153 were informative, while D13S156 could not be used for linkage analysis in this family. The first lane of each gel is DNA size marker VIII (Roche)
|
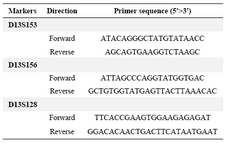
Table 1. The primer sequences used for amplification of microsatellites markers
|
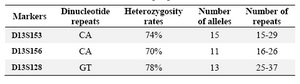
Table 2. The results of analyzing the three microsatellites markers in normal people
|
|