The Quality and Quantity of Nanoparticles Extracted from Human Adipose Tissue Derived-Mesenchymal Stem Cells
-
Karimi, Mobina
-
Department of Biology, SR.C, Islamic Azad University, Tehran,
-
 Heidari, Banafsheh
Department of Regenerative Medicine and Biotechnology in Wound Healing, Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran, E-mail: b.heidari@acecr.ac.ir
Heidari, Banafsheh
Department of Regenerative Medicine and Biotechnology in Wound Healing, Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran, E-mail: b.heidari@acecr.ac.ir
-
Jafary , Hanieh
-
Department of Biology, SR.C, Islamic Azad University, Tehran, Iran
-
Zandsalimi, Kavosh
-
Department of Medical Laser (MLRC), Medical Laser Research Center, Yara Institute, ACECR, Tehran, Iran
Abstract: Background: Nanoparticles, as small extracellular vesicles, are considered promising tools in tissue engineering and regenerative medicine. This study aimed to investigate the effects of different processing and culture condition on the quality and quantity of extracts derived from human Adipose-Mesenchymal Stem Cells (AD-MSCs).
Methods: AD-MSCs were proliferated in both the experimental and control groups. Nanoparticles were extracted from AD-MSCs-extracts and analyzed using SEM, TEM, DLS, Zeta potential, FTIR and BCA analyses. The morphological characteristics (shape, size, distribution, surface topography, and agglomeration/aggregation), structural appearance (poly-disperse intensity, colloidal particle behavior, surface charge, and stability), chemical properties (functional groups and ionic interactions) and total protein concentration were detected in the extracted nanoparticles. Additionally, the morphological characteristics, apoptosis, mitochondrial oxidoreductase activity, and migration potential of AD-MSCs in both groups were evaluated using acridine orange staining, MTT, and scratch assays.
Results: In the experimental group, 100% of the nanoparticles had a diameter of 112.8±25 nm, with the most frequency of 111.4 nm. However, in the control group, 72% of nanoparticles had a diameter of 350.2±43.6 nm with the highest frequency of 339.8 nm (p≤0.05). The Z-average, Poly-disperse intensity, and electrostatic stability of nanoparticles in the control and experimental groups were 171.9 nm, 0.727 and -0.000011 cm2/Vs vs. 103.7 nm, 0.205 and 0.000481 cm2/Vs, respectively (p≤0.05). In the experimental group, Zeta potential was -61.8 mV, which is in the range of ζ >-30mV. Although, Zeta potential in the control group was -1.5 mV, which is in the range of -30 mV <ζ <30 mV (p≤0.05). Total protein concentrations in the control and experimental groups were 11 and 41%, respectively (p≤0.05).
Conclusion: Nanoparticles derived from AD-MSCs have high therapeutic applications in tissue engineering and regenerative medicine.
Keywords:
Acridine orange
,
Control groups
,
Fourier transform infrared
,
Immunologic factors
,
Nanoparticles
,
Obesity
,
Regenerative medicine
,
Regenerative medicine
,
Spectroscopy
,
Static electricity
,
Tissue engineering
Introduction :
Cell-free therapy has been widely used in treating various diseases, including immunological and neurological disorders, degenerative diseases, liver failure (fibrosis, cirrhosis, and ischemia-reperfusion injury), cardiovascular diseases (myocardial infarction and ischemia), renal disorders, pulmonary deficiencies, and skin injuries. Cell derivatives not only have numerous clinical applications in treating various diseases but are also used as biomarkers for early rapid diagnosis of abnormalities/diseases. There are several studies demonstrated that the paracrine factors secreted by various cell types are specialized and unique in their types, concentration of biologically active compounds and functional properties. Therefore, the identification of these factors can specifically identify the type of cells (cancer cells or normal cells) and their functional nature, aiding in their early diagnosis and targeted treatment 1-4.
The bioactive paracrine factors in cell derivatives regulate many biological and pathological processes; such as establishing intercellular communication, altering/modifying cell signaling, and inducing the pathways related to immune responses, inflammation, differentiation, metastasis, migration, angiogenesis, apoptosis, etc. 5-7. One of the most important bioactive components in cell derivatives is exosomes with a diameter of 40-160 nm and a density of 1.13-1.19 g/ml. These microvesicles have a phospholipid bilayer membrane and contain various biological compounds such as lipids, DNA, mRNA, miRNA, various types of proteins such as heat shock proteins (HSP70 and Hsp90), enzymes, membrane transporters (Annexin and GTPases), and tetraspanins (CD9, CD63, CD81, and CD82). Exosomes are non-toxic, biocompatible, and biodegradable. They can transfer from the blood-brain barrier or blood-testicular barrier and exert therapeutic effects on the CNS and gametes. In addition, they can specifically bind to the target cells due to their surface receptors. The other advantages of exosomes include high stability in biological fluids, strong immunomodulatory properties, long-term storage capability without the need for various cryoprotectants, and the potential to engineer them for specific purposes such as producing synthetic exosomes 1,4,5,8,9. Another advantage of exosomes is their portability, affordable and ease of transport, which eliminates the need for specialized carriers or special laboratory-medical preparations. These therapeutic nanoparticles can be efficiently frozen at -80°C with or without non-penetrating cryoprotectants such as trehalose or sucrose and thawed at 37°C while maintaining the therapeutic efficacy. This allows exosome therapy to expedite treatment for acute diseases requiring immediate intervention such as cerebral ischemia, myocardial infarction, or other rapidly progressive diseases because the frozen exosomes can immediately be thawed and injected into the patient. In contrast, cell therapy requires cells to be cultured and reactivated post-thaw, along with viability assessment before transplantation 4,5,8-10.
Exosomes are divided into three groups based on size heterogeneity, including ExoA, ExoB, and ExoC. ExoA has dimensions of 40-75 nm, ExoB has a size of 75-100 nm, and ExoC has a size of 100-160 nm 5,11. Based on their content heterogeneity, exosomes are categorized into three groups, including Exo 1, Exo 2, and Exo 3. The first group, Exo 1, contains CD63 molecular marker and protein content of "X". Exo 2 has CD9 biomolecular, nucleic acids and protein content of "Y". Exo 3 includes CD81 molecular marker and protein content of "Z" 5,11,12. In terms of functional heterogeneity, exosomes are also divided into three groups:
Exo α, Exo β, and Exo γ. Exo α imparts pro-survival signals on recipient cells. Exo β modulates the apoptotic process and Exo γ imparts immunomodulation on recipient cells 5,11-13.
This study aimed to investigate the effect of culture conditions and processing on the quality and quantity of nanoparticles isolated from human Adipose-Mesenchymal Stem Cells (AD-MSCs). For this purpose, the effect of different culture conditions on the morphological characteristics (shape, size, distribution, surface topography, and agglomeration/aggregation), structural appearance (poly-disperse intensity, colloidal particle behavior, surface charge, and stability), chemical properties (functional groups and ionic interactions) and total protein concentration of nanoparticle in the cell extracts using SEM, TEM, DLS, Zeta potential, FTIR, and BCA analyses were examined. Additionally, the cell count, apoptosis, mitochondrial oxidoreductase activity, and migration potential of AD-MSCs were detected using microscopic examination, acridine orange staining, MTT, and scratch assay.
Materials and Methods :
The study was approved by the Research Ethics Committee of the Islamic Azad University, Science and Research Unit, Tehran (IR.IAU.SRB.REC. 1403.082). Human adipose tissue (lipoaspirate) was obtained from healthy donors according to inclusion criteria. Donor inclusion criteria included age 45 and 55 years, no drug addiction, metabolic diseases, and liver disorders, as well as, absence of viral (HIV-1/2 Ab, HBV, HCV, syphilis and HPLV), mycoplasma, fungal, and bacterial infections. Except where otherwise indicated, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Collection of adipose tissue and its macroscopic and microscopic evaluations: Following liposuction of abdominal fat tissue using 4 mm cannulas, the adipose tissues were transferred to the laboratory on ice (4°C) for 20 min. The samples were prepared and evaluated using a protocol previously described by Heidari et al 14,15 with minor modifications. In the macroscopic evaluation, adipose tissues were examined for color, volume, density, and the presence of hematoma, hemorrhage, contusion, cysts and any abnormality. For histomorphological evaluation, 2 mg of adipose tissue was sent to the pathology laboratory in 25 ml of 10% formalin. Three paraffin blocks were prepared and 30 histological glass slides, each 5 μm thick, were prepared from paraffin blocks using a microtome (10 glass slides/paraffin block). Any evidence of necrosis, cysts, inflammatory cell invasion, calcification, fibrosis, cell rupture and ECM disintegration were detected using H&E staining. Each slide was evaluated separately by two blind pathologists and at least 5 fields were examined per slide.
Isolation and validation of mesenchymal stem cells derived from adipose tissue: After macroscopic and microscopic evaluations, adipose tissues were washed three times with warm Phosphate Buffered Saline (PBS) containing 100 U/ml penicillin, and 100 mg/ml streptomycin at 1200 rpm for 10 min. Then, the tissues were digested by a two-step enzymatic digestion method using collagenase type I (1 mg/ml) and trypsin-EDTA (0.25/1 mM) for 45 min at 37°C. The digested suspension was filtered through 70 µm nylon mesh and centrifuged at 1200 rpm for 10 min. Cell suspension was cultured in DMEM/F12 medium containing 100 IU Penicillin, 10% FBS and 100 μg/ml of streptomycin in an incubator at 37°C, 80% humidity and 5% CO2 for 3-5 days. After 70-80% confluency, the attached cells were trypsinized using trypsin-EDTA and cultured in the same condition at 37°C, 80% humidity and 5% CO2 (Passage 1). In the study, adipose tissue-derived MSCs were passaged 3 times and confirmed using flow cytometry, which confirmed the expression of CD29, CD14, CD45, CD34, CD105, and CD44 markers. At last, the confirmed AD-MSCs in passage 4 were frozen at a density of 9×105 cells in cryovials containing freezing medium (50% DMEM, 10% DMSO, 40% FBS) at -196°C.
Study design: The frozen AD-MSCs were thawed at 37°C and propagated at a cell density of 5×106 cells/ml in DMEM/F12 culture medium composed of 10% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin at 37°C, 80% humidity and 5% CO2. The adherent elongated MSCs displayed a homogeneous fibroblast-like morphology with spindle or triangular-shaped cell bodies, and large elliptical nuclei. The nonadherent cells were removed after 12 hr during the medium change. Then, the 25 cm2 culture flasks were divided randomly into two groups, including the control and experimental groups.
Extraction of nanoparticles in different groups: AD-MSCs in the experimental groups were incubated in DMEM/F12 composed of 10% KnockOut serum (Exo-free serum), 100 mg/ml streptomycin and 100 U/ml penicillin at 37°C, 22% O2, and 5% CO2 for 48 hr. Then, the supernatant was collected and centrifuged at 300 g, 2000 g, and 10000 g for 10 min, 10 min, and 30 min, respectively. The extracted media were passed through a 0.22 µm nylon mesh and centrifuged twice at 100000 g for 70 min. The pellet was processed in 400 μl PBS and stored at -80°C until use. In the control group, AD-MSCs were propagated with fresh culture medium containing DMEM/F12, 10% FBS, 100 mg/ml streptomycin and 100 U/ml penicillin for 48 hr in an atmosphere of 5% CO2, 37°C, and 22% O2. After 48 hr, the supernatant were collected and centrifuged at 300 g, 2000 g, and 10000 g for 10 min, 10 min, and 30 min, respectively. The extracted media were passed through a 0.22 µm nylon mesh and stored at -80°C until use.
The impact of culture condition on AD-MSC characteristics: The study evaluated how culture conditions affect the apoptosis, migration, and viability rate of AD-MSCs. To evaluate apoptosis using acridine orange staining, AD-MSCs were suspended at a density of 5×105 cells/well in a 6-well culture plate. In the experimental group, the cells were incubated in a culture medium composed of 1% KnockOut serum (Exo-free serum), 100 mg/ml streptomycin, and 100 U/ml penicillin at 37°C, 5% CO2, 5% O2, and 80% humidity for 24 hr. The control group received DMEM/F12 supplemented with 10% FBS, 100 IU/ml penicillin, 100 µg/ml streptomycin at 37°C, 5% CO2, 22% O2, and 80% humidity for 24 hr. Then, 100 µl acridine Orange diluted in 100 ml of PBS was added to each well for 10 min at 37°C in the dark. After this time, the plates were washed twice with PBS and examined using a fluorescence microscope.
To investigate the migration of AD-MSCs, the scratch test was performed in both the control and experimental groups. For this purpose, AD-MSCs were cultured at a density of 5×105 cells/well in a 6-well culture plate using a medium with 1% KnockOut serum in the experimental group and 10% FBS in the control group for 24 hr. After 80-90% confluency, the scratch was created in the cell monolayer with a yellow pipette tip (10-100 µl). Images were captured immediately (0 hr), 6 hr, 12 hr, 18 hr, and 24 hr post-scratch and analyzed using ImageJ software.
The mitochondrial oxidoreductase activities of AD-MSCs in both the control and experimental groups using MTT assays were investigated. Approximately, 1×104 AD-MSC were propagated under different conditions in each well in 96 well plates and incubated for 24 hr. Then, 100 µl MTT solution (0.5 mg/ml) was added to each well for 3 hr in the dark. MTT solution was carefully aspirated and 100 µl DMSO was added to dissolve the formazan precipitate. The absorbance was detected at 570 nm in a plate reader.
The effect of culture condition on the adipose tissue-derived MSCs-nanoparticles: To characterization of nanoparticles extracted from AD-MSCs, the morphological characteristics (shape, size, distribution, surface topography, and agglomeration/aggregation), structural appearance (poly-disperse intensity, colloidal particle behavior, surface charge, and stability), chemical properties (functional groups and ionic interactions) and total protein concentration of nanoparticle in different culture conditions using SEM, TEM, DLS, Zeta potential, FTIR, and BCA analyses were evaluated.
Morphological characteristics of nanoparticles in different conditions: Shape, size, distribution, surface topography, and agglomeration/aggregation of nanoparticles in both the control and experimental groups were detected using SEM and TEM. To SEM analysis, the extracted nanoparticles were fixed with paraformaldehyde 2% and diluted with distilled water. Then, 5 µl exosomes were transferred onto silicon chips and placed in acetone for one min to dehydrate and dry. After washing and drying, the nanoparticles were analyzed by an electron microscope at a voltage of 30 KV. For TEM evaluation, the nanoparticles from different groups were fixed using glutaraldehyde 1%. The drop was placed on a carbon-coated grid and dried for 2 min at room temperature. Then, the slides were washed twice with sterile PBS and stained with uranyl acetate 1% for 16 min. The corresponding images in both groups were recorded using a Philips CM-120 microscope (made in the Netherlands) with a voltage of 120 KV.
Structural appearance of nanoparticles extracted from the control and experimental conditions: Structural characteristics of nanoparticles including poly-disperse intensity, colloidal particle behavior, Z-average, surface charge, and stability in both the control and experimental groups were detected using DLS and Zeta potential analysis. For this purpose, 100 μl of extracted nanoparticles in different groups were mixed with 400 μl of filtered PBS (1:5 ratio) and sonicated on ice for 20 min. Then, the sample was placed in a SZ-100 device (Horiba Company, Japan) and a graph related to the nanoparticles was recorded along with the zeta potential (surface charge present in the nanoparticles and their electrostatic stability).
Chemical properties of extracts from AD-MSCs in the control and experimental conditions: FTIR analysis revealed the functional groups and chemical composition of extracts through their infrared absorption. Spectral investigation of nanoparticles in the extracts was obtained at 4000-4000 cm-1 using an AVATAR spectrometer (Thermo, USA). This analysis determined the biochemical composition of extracts, highlighting the similarities and differences in the molecular structure between the control and experimental groups.
Total protein concentration in the extracts from AD-MSCs: Total protein concentration was evaluated using a BCA protein assay kit (Thermo Scientific Pierce, Rockford, IL, USA). For this purpose, various volumes of the nanoparticles extracts (10, 20, 30, 40, and 80 ml) were centrifuged to collect samples with increasing nanoparticles doses. The protein in the samples were released using a specific protocol, and isolated nanoparticles were re-suspended in 200 μl of RIPA buffer containing a protease inhibitors cocktail, followed by sonication for 5 min. BSA standard and samples (25 μl) were transferred to a 96-well plate and working reagent (200 μl) was added to each well (in a 50:1 ratio of assay reagents A and B). The plate was incubated at 37°C for 30 min, before being analyzed with a spectrophotometer at 562 nm (Victor3, Perkin-Elmer, and Waltham, MA, USA).
Statistical analysis: The statistical software used in this study was SPSS 16 and GraphPad Prism 6. Quantitative data will be presented as mean and standard deviation (in case of non-normality from the mean and range) and qualitative data will be presented as frequency and percentage. T-tests and Wilcoxon tests will be used to compare the results between groups. A statistical significance level more than or equal to 0.05 is considered.
Results :
Proliferation and differentiation of AD-MSCs in different culture conditions: The microscopic evaluation of AD-MSCs in different groups is shown in figure 1. These cells were observed to be spindle-shaped (fibroblast-like) with fully extended invasion. Total concentration of AD-MSCs reached to 20, 30, 45, 60, 80 and 100% on days 1, 2, 3, 4, 5 and 6, respectively (Figure 1). The expression of CD29, CD14, CD45, CD34, CD105, and CD44 molecular markers on AD-MSCs are demonstrated in figure 2. AD-MSCs propagated in the control and experimental groups were able to differentiate into chondrocytes (metachromatic violet), osteocytes (reddish-brown calcium deposits) and adipocytes (yellow-orange fatty vacuoles) (Figure 3). There were no significant differences in the total counts, differentiation capacity, and migration of AD-MSCs between the control and experimental groups (Figures 3 and 4; p>0.05).
Effect of culture conditions on the mitochondrial reductase activity and apoptosis and of AD-MSCs: The apoptotic rate of AD-MSCs in the control and experimental groups is shown in figures 5A, and 5B. According to the acridine orange staining, AD-MSCs demonstrated green fluorescent dyes, indicating no apoptosis of AD-MSCs in different groups. No statistically significant difference was observed in the mitochondrial reductase activities of AD-MSCs between the control (97.5%) and experimental (95.9%) groups (Figure 5C; p>0.05).
Size, shape and surface topography of extracted nanoparticles in different culture conditions: The morphological characteristics of nanoparticles derived from AD-MSCs are demonstrated in figure 6 and Histogram 1. Nanoparticles in the different groups are observed as spherical bodies with a uniform and consistent distribution using SEM. There was a statistical difference in the size of extracted nanoparticles in the control and experimental groups (p≤0.05). In the control group, the greatest number of nanoparticles was in the range of 100-300 nm, while in the experimental group, the highest frequency of nanoparticles was in the range of 60-100 nm (p≤0.05). SEM showed that the extracted nanoparticles in the experimental group were in the range of exosomes (40-160 nm), while most na noparticles in the control group were in the range of micro-vesicles or ectosomes (200-1000 nm) (Figure 6; Histogram 1; p≤0.05).
Aggregation and dispersion of extracted nanoparticles in different culture conditions: The effect of culture conditions on the structure characteristics and dispersion/aggregation of nanoparticles from AD-MSCs is shown in figure 7. In the control group, the variation in size and dispersion of nanoparticles is demonstrated using TEM analysis. The most dispersion and aggregation of nanoparticles are in the control group, while this amount is significantly lower in the experimental group (p≤0.05; Figure 7). Also, the agglomeration of nanoparticles in the control group was significantly higher than that of experimental group (Figure 7; p≤0.05).
Zeta potential and electrostatic stability of nanoparticles in different culture conditions: The Zeta potential and electrostatic stability of nanoparticles in both the control and experimental groups are shown in Figure 8. Zeta potential curves in the control and experimental groups have a narrow peak, indicating that the surface charges of nanoparticles are uniform. There are statistically significant differences in the Zeta potential and electrostatic stability of nanoparticles between the control and experimental groups (p≤0.05). In the experimental group, Zeta potential was -61.8 mV, which is in the range of ζ >-30 mV, so the nanoparticles were stable. On the other hand, the Zeta potential in the control group was -1.5 mV, which is in the range of -30 mV <ζ <30 mV (p≤0.05). So, the particles were unstable and tended to aggregate (Figure 8). In addition, the electrostatic stability of nanoparticles in the control and experimental groups are -0.000011 cm2/Vs and 0.000481 cm2/Vs, respectively (p≤0.05).
Poly-disperse intensity and Z-Average of nanoparticles in different groups: A statistically significant difference is observed in the Poly-disperse Intensity (PI), dimensions, and Z-average of nanoparticles between the control and experimental groups (Figure 9; p≤0.05). According to figure 9, in the experimental group, 100% of the nanoparticles had a diameter of 112.8±25 nm, with the most frequency of 111.4 nm. However, in the control group, 28% of the nanoparticles had a diameter of 35.6±3.2 nm with the highest frequency of 35.7 nm, and the remaining 72% of nanoparticles had a diameter of 350.2±43.6 nm with the highest frequency of 339.8 nm (p≤0.05; Figure 9). The PI and Z-average of nanoparticles in the control and experimental groups are 171.9 nm and 0.727 vs. 103.7 nm and 0.205, respectively (Figure 9).
Chemical properties and functional groups of extracts in the control and experimental conditions: The chemical composition and functional groups of nanoparticles extracted in the control and experimental conditions are shown in figure 10. There was no significant difference in the peaks, wavelengths, and their interpretations between the control and experimental groups (p>0.05). The peaks, wavelengths, and their interpretations, particularly the relevant functional groups, are detailed in Figure 10. FTIR analysis indicates that the extracted nanoparticles contain various compounds, including proteins (according to amide I and II peaks), lipids (due to aliphatic C-H peaks), glycoproteins or phospholipids (according to C-O or P-O peaks), and nucleic acids (due to the presence of P-O peak) (Figure 10).
Total protein concentration in the extract derived from AD-MSCs: The absorption rate of standard solutions and total protein contents in the control and experimental groups are demonstrated in figure 11. There was a statistical significant difference in the total protein concentration between the control and experimental groups (p≤0.05). The amount of total protein in the experimental and control groups are measured as 41.09444, and 11.56953%, respectively (Figure 11).
Discussion :
In the present study, nanoparticles with a diameter of 112.8±25 nm (mode: 111.4 nm) were achieved in the experimental group. The PI, Z-average, and Zeta potential of nanoparticles were 103.7 nm, 0.205, and -61.8 mV, respectively. In addition, the electrostatic stability of nanoparticles derived from AD-MSCs was 0.000481 cm2/Vs, Consistent with our results, Safwani and his colleagues in 2016 evaluated the effect of different culture conditions on propagation of MSCs derived from adipose tissue. This study aimed to introduce the alternative culture conditions that provide a better and safer environment for cell growth. They divided AD-MSCs into four experimental groups including: 1) 21% oxygen with FBS, 2) 21% oxygen without FBS, 3) 2% oxygen with FBS and 4) 2% oxygen without FBS. Microscopic examination showed that the AD-MSCs in all experimental conditions had spindle-shaped and fibroblast-like morphology. They expressed the positive molecular markers such as CD105, CD73, CD90 and CD44 more than 90% of cells. However, the hematopoietic cell biomarkers including CD34, CD19, CD14 and CD45 do not express on the cell surface. They presented a significant increase in the expression of CD14 and CD34 in the cells cultured in the serum starvation group with 2% oxygen 16. Kim et al in 2021 evaluated the nanoparticles extracted from human umbilical cord-MSCs. They also investigated the expression of pro-inflammatory factors in the cell extracts depending on the cellular status. Additionally, the wound healing process and angiogenesis activities were investigated using nanoparticles extracted from a human umbilical cord-MSCs. Human umbilical cord-MSCs were randomly divided into two the control and experimental groups. In the control group, MSCs were cultured in a low-glucose medium supplemented with 10% FBS and 1% antibiotic-antifungal in a humidified incubator with 5% CO2 at 37°C. However, the cells in the experimental group propagated in a medium containing 1% antibiotic-antifungal mixture under serum starvation conditions. The expression of CD63 and CD81 biomarkers in the nanoparticles extracted from umbilical cord-MSCs were examined using Western Blot analysis. In this study, FTIR analysis revealed an amide I absorption band at 1650 cm⁻¹, an amide II absorption band at around 1540 cm⁻¹, an absorption for the lipid acyl chain between 2860 cm⁻¹ and 2940 cm⁻¹, and an absorption of the carbonyl ester group around 1740 cm⁻¹, which are directly related to all subpopulations of exosome. The expression of HGF and bFGF increased in the control group compared to the experimental group. Also, the release of pro-inflammatory cytokines was significantly reduced in the control condition compared to the experimental group 17. One of the most important controversial point in the above article is the use of Fetal Bovine Serum (FBS) as a supplement of culture medium in their article. It is well documented that the FBS contains a high concentrations of nanoparticles, microvesicles, unknown extra cellular particle, proteins, growth factors, and unknown components. Therefore, isolation of exosomes from the extract derived from human umbilical cord-MSCs may be related to FBS components and not MSCs extract.
In accordance with our results, Wei et al in 2020 isolated nanoparticles from the human amniotic epithelial cells that propagated in a medium containing high-glucose DMEM (33 mmol/l glucose) supplemented with 10% FBS and 2% penicillin/streptomycin at 37°C, 5% CO2, and 80% humidity. Similar to our protocol, when cell confluence reached 70-80%, the cells incubated with DMEM containing 10% exosome-free FBS for 48 hr and centrifuged for extraction of nanoparticle from supernatant. In this study, the nanoparticles extracted from the above protocol were presented a cup shape (spherical morphology) with a mean diameter of 105.89±10.36 nm that expressed the CD9, CD63, TSG101, and Alix molecular markers. Application of these nanoparticles in treating of diabetic ulcers significantly promoted cell proliferation, enhanced the migration of fibroblasts, suppressed the production of cytokines associated with inflammation, and facilitated the angiogenic activity of cells. These researchers confirmed the biological effects of nanoparticles derived from amniotic epithelial cells on diabetic wound healing 18.
In 2024, Misaghian et al investigated the effect of nanoparticles derived from human Wharton's jelly- MSCs that were treated by polyinosinic-polycytidylic acid sodium salt on regulatory T cells. Then, the peripheral blood mononuclear cells were co-cultured with isolated nanoparticles for 6 days. In this study, the nanoparticles derived from treated Wharton's jelly-MSCs had an average size of 50 to 200 nm, with the most frequency of 132 nm. They confirmed that the 93% of purified nanoparticles had spherical morphology with an efficient integrated membrane and a diameter <132 nm. The exosomes derived from treated Wharton's jelly-MSCs significantly increased the frequency of CD4+CD25+Foxp3+egulatory T cells 19. Dang et al also found that the nanoparticles derived from human umbilical cord-MSCs had a near-spherical shape with average size of 127.7 nm. These small size microvesicles were positive for CD63 and CD81 molecular markers 20.
In present study, nanoparticles with a higher negative zeta potential (-61.8 mV) indicate greater electrostatic stability (0.000481 cm2/Vs) and reduced aggregation risk. To determine the stability of extracted nanoparticles in biological contexts, detection of zeta potential and electrostatic stability are necessary. These techniques can predict the colloidal behavior and tendency of nanoparticles for aggregation or agglomeration. The nanoparticles with a higher negative zeta potential (more negative surface charge) exhibit greater electrostatic stability and have a lower tendency to aggregation and agglomeration. However, a positive Zeta potential (less negative charge) indicates a greater chance for disruption and agglomeration, which potentially leads to adverse biological reactions and decreased therapeutic effectiveness.
Conclusion :
Nanoparticles secreted from AD-MSCs, are considered promising therapeutic tools in tissue engineering and regenerative medicine. Nucleoproteins in the extracted nanoparticles in the experimental group was approximately 4 times higher than in the control group, which may indicate a greater therapeutic potential of these nanoparticles.
Ethical approval :
The study was approved by the Research Ethics Committee of the Islamic Azad University, Science and Research Unit, Tehran (IR.IAU.SRB.REC. 1403.082).
Acknowledgement :
The authors would like to thank the Yara Institute for technical support, ACECR, Tehran, and Dr. Kamali for assistance with the statistical analysis.
Conflict of Interest :
The authors declared no conflict of interest.
Figure 1. Proliferation of AD-MSCs at passage 3 on days 1 (A), 2 (B), 3 (C), 4 (D), 5 (E) and 6 (F) post culture initiation, magnification 40 X.
|

Figure 2. The expression of CD44 (A), CD29 (B), CD105 (C), CD14 (D), CD45 (E), and CD34 (F) biomarkers on AD-MSCs.
|
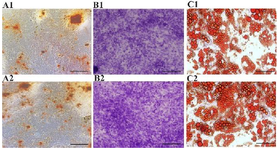
Figure 3. Differential potentials of AD-MSCs into osteocytes (A1, A2), chondrocytes (B1, B2) and adipocytes (C1, C2) in the control (A1-C1) and experimental (A2-C2) groups, magnification 40X.
|
Figure 4. Migration of AD-MSCs at 0 hr (A, a), 6 hr (B, b), 12 hr (C, c), 18 hr (D, d) and 24 hr (E, e) in the control (A-E) and experimental (a-e) groups, magnification 40X.
|
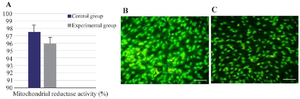
Figure 5. Mitochondrial reductase activities (A), and apoptosis of AD-MSCs in the control (B) and experimental (C) conditions with a magnification of 40X.
|
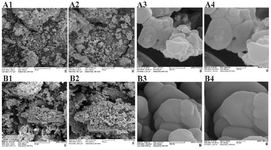
Figure 6. Extracted nanoparticles in the experimental (A1-4) and control (B1-4) groups with magnifications of 50 μm (A1, B1), 20 μm (A2, B2), 500 nm (A3, B3) and 200 nm (A4, B4).
|
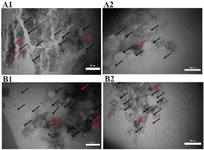
Figure 7. Aggregation, dispersion, and agglomeration of extracted nanoparticles in the control (A) and experimental (B) groups with magnifications of 50 nm (A1, B1) and 100 nm (A2, B2). Black arrows demonstrate the typical nanoparticles and red arrows are agglomerated and aggregated nanoparticles.
|
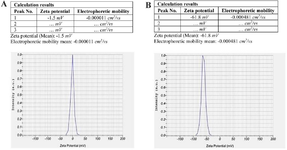
Figure 8. Zeta potential and electrostatic stability of nanoparticles extracted from AD-MSCs in the control group (A) and experimental group (B).
|

Figure 9. Poly-disperse intensity and Z-average of nanoparticles extracted from AD-MSCs in the control group (A) and experimental group (B).
|

Figure 10. The functional groups, wavelengths, and their interpretations in the extracts of the control (A) and experimental (B) conditions using FTIR analysis.
|

Figure 11. Absorption rate and total protein concentrations of AD-MSCs extracts in different groups.
|
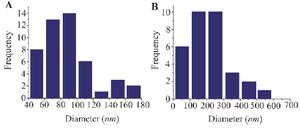
Histogram 1. Frequency and distribution of nanoparticles in the experimental (A) and control (B) groups.
|
|