Non-gynaecological Applications of Menstrual-derived Stem Cells: A Systematic Review
-
 Galea, Claire
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta, Tel: +00 356 23401892; E-mail: claire.galea.15@um.edu.mt
Galea, Claire
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta, Tel: +00 356 23401892; E-mail: claire.galea.15@um.edu.mt
-
Riva, Nicoletta
-
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
-
Department of Pathology, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
-
Calleja-Agius, Jean
-
Department of Anatomy, Faculty of Medicine and Surgery, University of Malta, Msida, Malta
Abstract: Menstrual-derived Stem Cells (MenSC) are a potential novel source of mesenchymal stem cells. There is an increased interest in investigating the therapeutic potential of MenSC due to the various advantages they exhibit, when compared to other types of stem cells. MenSC are obtained non-invasively from menstrual blood. Thus, collection of MenSC is simple, reproducible and can be carried out periodically, with minimal complications. MenSC are present in abundance, are highly proliferative, exhibit a low immunogenicity and lack ethical issues. MenSC have shown the ability to differentiate into several lineages. The therapeutic potential of MenSC in non-gynaecological applications has been investigated in wound healing, neurological, musculo-skeletal, cardiovascular, respiratory, and liver disorders, as well as in diabetes and cancer. Human clinical trials are limited. To date, therapeutic efficacy and safety have been reported in patients with Avian influenza A subtype H7N9, COVID-19, congestive heart failure, multiple sclerosis and Duchene muscular dystrophy. However, further clinical trials in humans should be conducted, to study the long-term therapeutic effects of these stem cells in various diseases and to further explore their mechanism of action. This systematic review focuses on the application of MenSC in non-gynaecological diseases.
Introduction :
A novel source of mesenchymal stem cells was identified from menstrual blood in 2007 and they were called Menstrual-derived Stem Cells (MenSC) 1,2. Increased interest was shown towards MenSC due to their abundance, continuous source, non-invasive collection via menstrual cups, low immunogenicity, high proliferation, differentiation ability and lack of ethical issues 3-5. The mechanisms of action of MenSC are by differentiation into target cells, immunomodulation, paracrine cytokine secretion, migration and engrafting into injured sites 6. MenSC can differentiate into several lineages including endothelial, osteogenic, chondrogenic, adipocytic, pancreatic, hepatic, respiratory epithelial, cardiomyocytic, and neurocytic line-ages 1,7-10. MenSC have shown potential therapeutic application in various non-gynaecological disorders including wound healing 11-14, neurological 15-17, musculoskeletal 18,19, cardiovascular 5,20,21, respiratory 5,20,22 and liver 23,24 diseases, as well as in diabetes 25,26 and cancer 27-31. Several studies also considered the gynaecological applications of MenSC, such as in ovarian-related diseases 32,33, endometrial injury 34,35 and Asherman syndrome 36,37.
Meng et al 38 were the first authors to report the presence of stem cells in menstrual blood which were referred to as endometrial regenerative cells. In the literature, various nomenclatures were used to refer to MenSC which are listed in table 1.
To obtain MenSC, menstrual blood from healthy women is collected using menstrual cups. The exclusion criteria used in various studies were: vaginal infection history, non-steroidal anti-inflammatory drugs, corticosteroids and oral contraceptives use within the last 3 months, infections such as human immunodeficiency viruses, hepatitis C virus and hepatitis B virus, autoimmune diseases, diabetes, endometriosis and malignancies 63-66. Menstrual blood for the isolation of MenSC should be collected on the day with the heaviest blood flow 65-66 usually the second day 64,67-69. Around 5 ml volume of blood will provide sufficient stem cells. The age range of the female participants in the studies ranged from 18 years to 45 years old with the most common age group being between 22 and 30 years 56,64-67.
The collected menstrual blood is transferred in Phosphate Buffered Saline (PBS), amphotericin B, streptomycin, penicillin and Ethylenediaminetetraacetic acid (EDTA) 38,44,56,70. The temperature of the collected sample is maintained at 4oC and is trans-ported within 24 hr upon collection to the laboratory for processing 70. The Ficoll-Paque Density Gradient Centrifugation (DGC) method is used to separate menstrual blood mononuclear cells and then the separated cells are washed with PBS. The isolated cells from the middle white layer of endometrial cells are cultured in T25 flask containing modified Eagle’s medium, 1% penicillin/streptomycin with high glucose, 1% amphotericin B, 15% Fetal Bovine Serum (FBS) and 1% glutamine at 37oC to obtain adherent cells 56,71. The next day, the media are changed so that non-adherent cells are washed out, 0.05% trypsin-EDTA is used to detach adherent cells, the cells are then counted and subcultured. The culture media are changed twice a week and are incubated at 37°C with 5% CO2 and saturated humidity 71. At 7 to 10 days of incubation when the cell confluency reaches 70%, the culture medium is removed and around 5 ml of pre-warmed trypsin-EDTA is used to detach the cells for 2 to 3 min in CO2 incubator. Trypsin is then neutralised by adding 10 ml media containing 20% FBS and the cells are gently collected. The cells are passaged and, after 2 to 3 passages, there is sufficient cells for differentiation experiments 65,66.
The DGC is the conventional method used for isolation of MenSC, however, in the buffy coat this method leaves flocculent membranes and karyocytes of the deciduous endometrium 38,58. Most of the MenSC clones are produced by the deciduous endometrium. Moreover, it was noted that menstrual blood clots would interwind with deciduous endometrium after DGC. In the study by Sun et al 72, a method for high yield isolation of MenSCs was tested which differs from the method described above. It was shown that MenSC remained in sedimentation after DGC. Red Blood Cell Lysis Buffer (RLBD) was used directly and compared to DGC. MenSC isolation was increased by RLBD. In this study, the DGC method was used but then from the buffy coat, the deciduous endometrium and karyocytes were transferred to a new tube and washed with PBS 72. These were then suspended in high glucose Dulbecco’s Modified Eagle’s Medium (DMEM) and transferred into new tubes with supernatant removal. Then, the red blood cell lysing buffer was added to suspend the sedimentation at room temperature for 3 to 5 min. RBC lysis was performed twice till most of the erythrocytes were lysed osmotically. Washing with PBS was done and then the suspension of the sedimentation in growth medium was incubated at 37°C with 5% humidified CO2. This method collected a higher number of MenSC than the conventional DGC 72.
High concentrations of Platelet Rich Plasma (PRP) affect MenSC culture duration. It was reported that the optimal PRP concentration for MenSC proliferation was 10% 73,74. Processing of menstrual blood up to 72 hr after collection does not cause property changes in the MenSC plastic adherence 44. For therapeutic application, MenSC must be expanded in culture so as to obtain enough cells for use. With increase of passage number, MenSC proliferation rate decreases gradually due to aging of MenSC 75. There is a change in morphology in highly passaged cells as they increase in size, lose the fibroblastic morphology and appear senescent. High passing of stem cells (P20) causes gene alteration which are involved in stress response, transcriptional regulation, development, cell proliferation and apoptosis. Moreover, at high passages there is karyotype alteration. Zemel’ko et al 76 reported that MenSC at 45 population doubling underwent aging. Meng et al 38 showed that MenSC maintained normal karyotype with no tumour development after 68 PD. During MenSC passages, pluripotency protein expression of octamer-binding transcription factor 4 (OCT-4) positive cells during the first passage was 97.5%, while when the twelfth passage was reached the positivity of OCT-4 positive cells was reduced to 19.4%. The study by Kahanmohammadi et al 51, reported that at the second and twelfth passage MenSC diploid phenotype was kept without any aberration in the chromosomes.
MenSC have the ability to differentiate into various lineages in the right conditions. Various studies have shown MenSCs ability to differentiate into osteogenic, adipocytes, chondrogenic, hepatocyte-like cells, germ-like cells, neural-like cells, cardiomyocytes and keratinocytes-like cells 38,56,77. In most of the studies, prior to differentiation of MenSC into different lineage, the ability of MenSC to differentiate into adipogenic, chondrogenic and osteogenic was assessed.
MenSCs under light microscopy displays a spindle shaped and fibroblast-like morphology 38,78-80. At low density platting, MenSC grow as large flat cells in a monolayer and fibroblast colony forming units are created. A spindled shaped fibroblastic morphology is observed when the cultured cells reached confluence. A homogenous cell population is noted upon approaching confluence. It is noted that this morphology persisted at later passages (P10) 79.
MenSCs express Cluster of Differentiation (CD) 9, CD29, CD44, CD73, CD90, CD105 and CD166 cell markers. From these markers, CD9, CD29, CD73, CD90 and CD105 are commonly expressed in Mesenchymal Stem Cells (MSC). MenSC do not express CD14, CD34, CD38, CD45 and CD117 81 nor haematopoietic stem cell markers such as CD34, CD45 and CD133 38. HLA-ABC was weakly expressed 81 while HLA-DR was not expressed indicating that MenSC have low immunogenicity 82. Both endometrial MSC and MenSC co-express CD140b and CD146 83-84 and these markers can be used to discriminate from endometrial stromal fibroblast (CD146-CD140b+) and endometrial endothelial cells (CD146+CD140b-) 85. Human telomerase reverse transcriptase is expressed by MenSC (38,51). Borlongan et al 86 reported expression of Stage Specific Embryo Antigen 4 (SSEA-4), homeobox protein NANOG and sex-determining region Y-box 2 (SOX2). It was noted that MenSC have different stem cell marker profiles which is dependent on the environment 86. This might be a reason why expression of SSEA-4 varies from different studies ranging from 0 87 to 19.4% 88. It was also speculated that the difference in stem cell marker profile of MenSC can also be due to other donor’s factors including age, contraception and environmental factors 77. MenSC are commonly identified by the expression of OCT-4 which an embryonic stem cell surface marker 38,51,56,58. OCT-4 is not expressed by endometrial MSC 89,90 or Bone marrow-derived Mesenchymal Stem/Stromal cells (BMSC) 91. The molecule perivascular sushi domain containing-2 (SUSD2) specifies self-renewal and multipotency of MenSC 84.
In the studies by Meng et al 38 and Wu et al 57 it was reported that MenSC obtained from healthy and young women could increase in number, as every 20 hr it increases to one doubling under optimal culture conditions. MenSC doubling rate is twice faster than that of BMSC which is estimated at 40-45 hr 38,57. MenSC have a high proliferation rate which is due to the elevated expression of Extracellular Matrix (ECM) and embryonic trophic factors 92. For future research, the high proliferation ability of MenSC is important, as usually cell therapy is dose-dependent and low passage cells are used. Moreover, no obvious chromosomal abnormality was observed when MenSC were expanded in vitro. MenSC could have a huge clinical application due to their high proliferation rate, genetic stability and pluripotency 38,57,58.
Development of stem cells is associated to the Wnt signalling pathway. The Wnt signalling can regulate target genes that are downstream to mediate stem cell differentiation and proliferation 93,94. Transcription in the Wnt signalling pathway is initiated by transcription factor β-catenin binding to the promoter site on the Wnt-responsive genes. Cytoplasmic β-catenin in unstimulated cells is phosphorylated by Glycogen Synthase Kinase 3 (GSK3). Inactivation of GSK3 occurs on binding of Wnt to Frizzled. Accumulation of cytoplasmic β-catenin occurs as a result of GSK3 inactivation and translocation to the nucleus to activate Wnt-responsive genes 94. In a study by Kazemnejad et al 9, the role of Wnt signalling on MenSC proliferation was investigated. Different concentrations of Lithium chloride (LiCl) were used, since LiCl decreases MenSC proliferation in a dose-dependent manner. The expression of β-catenin increased with increasing concentration of LiCl, while MenSC proliferation was suppressed. This study shows that Wnt signalling pathway plays a significant role in MenSC proliferation. Further studies are still required to investigate the Wnt signalling in MenSC proliferation and differentiation into various lineages 79. The aim of this systematic review was to evaluate the non-gynaecological applications of MenSC.
Materials and Methods :
A systematic search was performed within the electronic databases MEDLINE, EMBASE and "Cochrane Central Register of Controlled Trials (CENTRAL)" to identify all the articles on MenSC published in English language. The search was done from 2007 till August 2020, week 4, and used the MeSH/EMTREE terms and keywords reported in appendix 1, 2 and 3.
Results :
The total number of articles retrieved were 1295: 584 from MEDLINE, 690 from EMBASE and 21 from Cochrane Central Register of Controlled Trials (Figure 1). Three additional relevant articles were retrieved from other sources: one was published in a journal not indexed in MEDLINE (Ovid), EMBASE or CENTRAL" Chen et al 39, the other 2 were published in February 2021, Xu et al 62 and March 2021, Lu et al 57. Thus, they could not be retrieved from the databases search.
Clinical potential of MenSCs: The therapeutic potential of MenSCs has been investigated in both preclinical and clinical studies (Tables 2 and 3).
Wound healing: MenSC have shown the ability to differentiate into keratinocytes and epidermal layer cells which can overcome some of the limitations of keratinocytes isolation (such as low proliferation rate, time-consuming and difficult preparation procedures) (Table 4) 1,12,95. Hence, MenSC could be clinically used for treatment of dermatological lesions and skin transplant 12. MenSC improve chronic wound healing regeneration and repair through promotion of cell adherence, proliferation and differentiation 1,12,95. MenSC overexpress the migratory molecule chemokine receptor type 4 (CXCR4) which binds to stromal cell-derived factor 1 (SDF-1) expressed by intrinsic fibroblasts, thus their migratory activity is directed to the site of injury 96. MenSC showed increased fibroblast migration under both basal and pro-inflammatory conditions. The hypoxic agent deferoxamine stimulated higher expression of Hypoxia-Inducible Factor (HIF)-1α in MenSC which displayed a higher angiogenic potential and increased factor expression [including Platelet Derived Growth Factor (PDGF), Vascular Endothelial Growth Factor (VEGF) and basic Fibroblast Growth Factor (bFGF)] 97. In turn, the migratory ability of fibroblast can be positively regulated by bFGF via the wnt/β catenin signalling pathway activation 98. MenSC upregulated the genes involved in angiogenesis and neovascularisation, such as angiopoietin 1 (ANGPT1), PDGFA, PDGFB and matrix metallopeptidase (MMPS), Extracellular Matrix (ECM) components, Elastin (ELN) and MMP10 that during remodelling of tissue helps to regulate collagen degradation 97. MenSC showed a lower expression of serpin family E member 2 (SERPINE2), which is an inhibitor of serine protease with anti-angiogenic function, and lower expression of the pro-inflammatory Interleukin (IL)-1β 97,99. Transforming Growth Factor (TGF)B2, a factor involved in keloid formation was downregulated in MenSC 97,100. Table 2 explains the findings observed in wound regeneration in vivo.
Central nervous system disorders: In an in vitro model of Parkinson’s Disease (PD), when human neuroblastoma SH-SY5Y cells with neurotoxin 1-methyl-4-phenylprudunuym (MPP+) were co-cultured in a culture medium obtained from MenSC, there was a significant increase in injured cell viability which could be due to increased anti-inflammatory cytokine release. After treatment, a decrease of pro-inflammatory cytokines caused by neurotoxin, which include IL-1β, IL-6, inducible Nitric Oxide Synthase (iNOS), Cyclooxygenase (COX-2) and Tumour Necrosis Factor (TNF)-α, was observed. The anti-apoptotic and antioxidant effects of MenSC culture medium could be caused by the presence of Neurotropin (NT)-3, NT-4, Brain-Derived Neurotrophic Factor (BDNF) and Epidermal Growth Factor (EGF) factors 101.
MenSC transplantation in a mice model of Alzheimer’s Disease (AD) showed amelioration of changes in the brain (Table 2). β-secretase (BACE1) and C-terminal fragment (β-CTF) levels, which are part of amyloidogenic pathway, were decreased. After MenSC treatment, the activated microglia were induced to express an alternative phenotype, instead of the neurotoxic phenotype characterised by anti-inflammatory cytokines secretions. IL-1β and TNF-α (pro-inflammatory factors) were significantly decreased, while the anti-inflammatory cytokine IL-4 was increased. There was also increase in messenger ribonucleic acid (mRNA) levels of cluster of differentiation (CD)-206, YM1, arginase-1 (Arg1) and found in inflammatory zone 1 (Fizz1), which are markers of anti-inflammatory alternative activation of macrophages 15.
Upon culturing of MenSC with neurons in a rat stroke model, protection against ischemia-induced cell death was observed. This could be due to elevation of trophic factors VEGF, BDNF and NT-3 102 which are reported to mediate therapeutic benefits of stem cell transplantation in various Central Nervous System (CNS) disorders 103,104. In rat ischaemic model, motor and neurological impairment was improved (Table 2). MenSC survived in the ischaemic striatal penumbra and the cells were detected even after 14 days since MenSC transplantation. Graft survival was better when administration of MenSC was given Intracranially (IC) rather than Intravenously (IV) (15% survival rate for IC vs. 1% for IV) 102.
For the first time, the study by Zhong et al 16 demonstrated the feasibility of MenSC administration in 4 patients suffering from Multiple Sclerosis (MS) (Table 3). This study shows promise for future use of MenSC in clinical settings, as no immunoreaction or ectopic tissue were observed at the site of injection 16. Rat with spinal cord injury treated with MenSC showed improved locomotor function, increased neuronal survival rate and preservation of tissue (Table 2). Neuronal survival rate increased in parallel with the increase of neuronal and glial cell markers neurofilament protein (NF)-200 and Microtubule-Associated Protein 2 (MAP-2) significant increase 105. MenSC transplantation with neural guidance conduits in a rat model showed significant improvement in the sciatic nerve injury. The reasons for this improvement might be due to the continuous secretion of neurotrophic factor and matrix protein by cells which assist in nerve repair 106. MenSC induce angiogenesis which is essential for neural cell repair and functional recovery after nerve injury 8,107.
Musculo-skeletal disorders: MenSC have a chondrogenic differentiation potential, which translates in the expression of Collagen 9A1 and SRY-Box Transcription Factor 9 (SOX9) at mRNA level, similarly to cartilage tissue 108. Insulin-like Growth Factor-Binding Protein 3 (IGFBP3) lacking MenSC were able to differentiate into chondrogenic lineage which could be due to higher sensitivity to the chondrogenic promotor factor, Insulin Growth Factor 1 (IGF-1) 109. Moreover, improvement was observed in osteochondral defect in vivo (Table 2) 110.
In mice models of Duchenne Muscular Dystrophy (DMD), MenSC exhibited extensive migratory ability and infiltration between muscular fibres. MenSC exhibited a myoblast differentiation, which could be an important finding for the future treatment of muscular diseases. Exposure of MenSC to 5-azacytidine pro-vides the highest desmin positive MenSC. MenSC do not express the genes Myogenic factor 5 (Myf5) and myosin heavy chain IIx/D (MyHC-IIx/d); however, the genes desmin, myogenic differentiation protein (MyoD) and dystrophin were expressed. MenSC administration improved dystrophin delivery and muscle regeneration 18. On the contrary, in the study by Ay et al 111. MenSC seeded on synthetic scaffold did not exhibit spontaneous differentiation and did not attach to the synthetic fibres 111. MenSC have been tested on a human individual with DMD and clinical improvement was observed and maintained for at least 2 years (Table 3) 112.
MenSC, when co-cultured with nucleus pulposus cells, show higher cell density and secretion of collagen II and aggrecan, which are important in functional maintenance of the intervertebral disc. Keratin 19 (KRT19), Carbonic Anhydrase 12 (CA12) and Forkhead box F1 (FOXF1) genes are markers for nucleus pulposus and they are significantly enhanced in co-culture with MenSC. This indicates that MenSC may have possible repair potential on nucleus pulposus 113.
MenSC injected in mouse limb ischemia models at the site of ischemia shows significant improvement in the ischaemic area, especially when compared to untreated mice (Table 2). The best results have been obtained in the group of mice that were injected and infused with MenSC. Intramuscularly (IM) injected mice showed better improvement than IV injected mice. MenSC transplantation, due to the factors secreted (VEGF, BDNF, NT-3) aid in the formation of small vessels in Critical Limb Ischemia (CLI) mice 19. There is an ongoing human phase I/II clinical trial of MenSC in CLI patients 114.
MenSC, when co-cultured with Achilles tendon cells, show the ability to differentiate into tenogenic cells. The differentiated MenSC express the genes for collagen I and collagen III which are important for maintenance of tendon function. The Achilles tendon markers Thrombospondin-4 (THBS4), Tenascin C (TENC) and scleraxis (Scx) are expressed and ECM is produced which is similar to the origin of Achilles tendon 115.
Cardiovascular disorders: To mimic myocardial infarction, MenSC have been cultured in vitro with hydrogen peroxide (H2O2)-induced injured rat cardiomyocyte cells. An increased cell viability rate and inhibition on the rate of apoptosis was observed. The altered mitochondrial function observed in H2O2 treated cells was restored when these cells were co-cultured with MenSC. MenSC co-culture decreased the levels of pro-apoptotic proteins such as Bcl-2-associated X protein (Bax) and increased the levels of anti-apoptotic protein B-cell lymphoma (Bcl)-2 which was observed in H2O2 treated cells. Moreover, the migration ability of H2O2 treated H9c2 cardio-myocytes was increased when co-cultured with MenSC, due to upregulation of N-cadherin 7. In vivo, improved cardiac function has been reported (Table 2) due to high in situ cardiomyogenic transdifferentiation ability of MenSC, showing clear striation and sarcomeric α-actinin 116. However, this is in contrast to the studies by Zhang et al 20 and Jiang et al 117 in which MenSC did not differentiate into cardiac lineage except for a small number of endothelial cells.
There is an ongoing clinical trial aiming to treat with MenSC 60 individuals with Congestive Heart Failure (CHF). Preliminary data on 17 patients showed no serious adverse event 1181. Another study reported a 74-year-old patient with CHF who received 5 shots of MenSC and cord blood via IV in a period of 7 months (Table 3) 119.
Respiratory system disorders: MenSC and MenSC derived exosomes have been administered to Bleomycin (BLM)-induced mice model and pulmonary fibrosis improved, as alveolar epithelial cell apoptosis were regulated 120,124. MenSC were principally captured and absorbed in the lungs when injected through the tail vein 120,122. The mice model showed significant improvement in pulmonary fibrosis (Table 2) 120-122. Zhao et al 121 reported that MenSC acted on the microenvironment of fibrosis with increase of anti-fibrotic factors, such as Hepatocyte Growth Factor (HGF) and MMP-9. The apoptotic factor Bax expression is reduced in lung tissue after MenSC treatment which might offer protection to alveolar epithelium cells from apoptotic damage. Secretion of TGF-β, which has a critical role in the changes observed in interstitial lung disease, has been shown to be reduced after MenSC treatment, thus potentially explaining why pulmonary fibrosis is alleviated after treatment 121.
In vivo and in vitro results in Acute Lung Injury (ALI) show that MenSC can migrate and stay in the injured area. In vivo improvement of lung function even at histological level was reported (Table 2). MenSC regulate the expression of cytokines to attenuate the inflammatory response 122,123. The mitogen Keratinocyte Growth Factor (KGF) expression increased after treatment with MenSC 123. The proliferative index has increased while caspase 3, an apoptotic index was reduced after treatment. Levels of proteins Phosphoinositide 3-kinase (PI3K), β-catenin and Vascular Endothelial (VE)-cadherin were increased after MenSC treatment, while downregulation of phosphoglycogen synthase kinase 3 beta (p-gsk3β), p-β-catenin and p-src was observed. The increase in VE-cadherin and β-catenin suggests that pulmonary microvascular permeability is improved after MenSC transplantation 122.
A clinical trial on humans with H7N9 infection was performed and even after 5 years from therapy amelioration of symptoms related to H7N9 infection have been reported (Table 3). However, the evaluation on the long-term effects of MenSC on all patients was not possible as some refused follow-up, which is a limitation of the study. The first results of a clinical trial conducted on 26 COVID-19 patients treated with MenSC (ChiCTR2000029606) 123 have reported amelioration of clinical manifestations (Table 3) 124. MenSC have already shown therapeutic potential in H7N9 patients who share similar clinical manifestation to COVID-19 patients 125.
Gastrointestinal disorders: MenSC treatment in animal models of ulcerative colitis has led to an amelioration of clinical symptoms, signs and histological alterations (Table 2). There is a reduction in macrophages-1 positive cell infiltration and intra-colon neutrophils in MenSC treated mice. The anti-inflammatory IL-4 and IL-10 were up-regulated, while the pro-inflammatory cytokines IL-2 and TNF-α were downregulated. CD11c+MHC-II+ DC were decreased in MenSC treatment, which indicates that MenSC exhibit an immunomodulatory effect in controlling colitis development. CD3+CD25+ active T cells have also been downregulated. In mice treated with MenSC there was an increase of CD4+CD25+ Foxp3+ Tregs, which is related to immune tolerance maintenance and autoimmune reaction downregulation, leading to reduction of colitis. CD3+CD8+ T cell levels were reduced, which improves cytotoxicity and immune system dysfunction 126. Programmed Death-Ligand 1 (PD-L1) which is important in immune suppression was expressed on MenSC cell surface and increased with stimulating factor concentration. This has contributed to reduced proliferation of inflammatory cells and effector function. It was reported that blockage of PD-L1 reduced MenSC efficacy in colitis treatment, since MenSC require PD-L1 for colitis attenuation. Inflammation in colitis is reduced by MenSC via PD-L1, by reducing neutrophils, CD3+ T cells and increasing alternatively activated macro-phages 127.
In animal models of liver fibrosis, MenSC have shown migratory ability into the liver lobule 128. The liver function tests have improved after transplantation (Table 2). In fact, there was rapid improvement of liver regeneration which indicate that the main mechanisms of liver repair by MenSC is unlikely to be by homing or by trans-differentiation. Instead, the likely mechanism of action is by trophic effect for hepatic tissue regeneration. Efficiency in liver regeneration by MenSC was the same in male and female mice models, indicating no gender difference in treatment outcomes, unlike other types of stem cells 129. MenSC can reduce lymphocyte antigen 6 complex locus G6D (Ly6G) positive cells in the liver. This gives an indication that MenSC act by suppressing infiltration of inflammatory cells to alleviate damage. MenSC was able to inhibit monocytes differentiation into dendritic cells to control liver injury. T cell accumulation is also inhibited as MenSC shows a reduced expression of CD4+ and CD8+ T cells 128. miR-122 hepatic levels correlate with the severity of liver damage and are downregulated after MenSC treatment 130. LX-2 human hepatic stellate cell proliferation is suppressed by MenSC, therefore indicating inhibition of stellate cells. There are increased levels of the anti-inflammatory Monocyte Chemoattractant Protein-1 (MCP-1), while the secretion of proinflammatory IL-6 is reduced by MenSC. This indicates protective factor properties during the initial stages of fibrogenesis. Although MenSC are able to ameliorate liver fibrosis, in order to reduce liver fibrosis there needs to be replenishing of hepatocytes 131. In vitro MenSC differentiate into hepatic lineage 10,132, however, in vivo only few cells differentiated. This could be due to the complex microenvironment 131. Hepatocyte marker genes are present in liver-like cells derived from MenSC including Albumin (ALB), Cytochrome (CY) P450, CYP3A4, CYP1A1, CYP7A1, Alpha Fetoprotein (AFP) and Cytokeratin (CK)-19 10,132,133. Other detoxification enzymes expressed are Glutathione S-transferase (GST)-A1, GSTA2 and GSTP1 133.
Endocrine disorders: The use of MenSC in vivo in diabetic mice models shows MenSC distribution in various organs but mainly in the pancreas, which could be due to the signals secreted by the injured area. Symptoms and pancreas morphology in diabetic mice model were improved (Table 2). Upon testing there was no insulin or human C-peptide detected, which indicates that MenSC did not differentiate into insulin-producing cells in the injured pancreas 25. MenSC were able to activate endocrine progenitor cells which reside in the duct, islet and exocrine tissue, and insulin increased indicating that MenSC promote the differentiation of pancreatic progenitor cells to β cells. Genes of β-cell development pancreas/duodenum homeobox protein 1 (pdx1) 25,134, forkhead box protein A2 (foxa2), nkx6.1, neurogenin-3 (ngn3), paired box 4 (pax4) and mafb were upregulated in MenSC treated mice which enhances β-cell generation. Genes associated with mature β-cell such as mafa, insulin and glucose transporter 2 (glut 2) were also upregulated 25.
Cancer: MenSC have been tested in vivo in animal models of different tumours, including glioma 30, Squamous Cell Carcinoma (SCC) 28, Hepatocellular Carcinoma (HCC) 135 and prostate carcinoma 29 (Table 2). MenSC were able to migrate to the tumour location, even when infected with adenovirus AD35-sTRAIL 30. It has been reported that MenSC interact with tumour cells via paracrine mechanisms 30,135. Tumour growth (weight and volume) was reduced after treatment 28,30,135. In HCC the proliferation rate of the tumour was reduced. Tet methylcytosine dioxygenase (TET)-1 and TET2 expression was increased in hepatocyte cells after co-culture with MenSC. This indicates that MenSC regulate DNA methylation via various methylation enzymes. MenSC can regulate epigenetic mechanisms found in HCC cells 135. Inhibition of angiogenesis was observed in prostate carcinoma 29 and SCC 28. In prostate tumour, after MenSC treatment, levels of VEGF and NF-κB activity were reduced 29.
Discussion :
An increased interest on these types of cells was shown by researchers due to the various advantages MenSC exhibit. MenSC are procured by an easy non-invasive method, they are abundant and a continuous source, they have low immunogenicity, high proliferation rate, differentiation ability into different lineages and lack of ethical issue 77. They can be obtained periodically and this is crucial for clinical efficiency. Sources such as bone marrow are obtained invasively and their number of culture passages are limited until the cells lose their stem cell potential and therefore MenSC are better in this aspect. Periodic collection can produce higher therapeutic dosages from the same genetical background. In addition, there are other functional properties of MenSC which can underlie specific uses. MenSC have a high proliferation rate and are able to differentiate in multiple cell lineages 56,77. Furthermore, MenSC have the ability to expand without losing the normality of the karyotype or tumorigenic potential up to 68 doubling 38,58. MenSC therapeutic potential has been studied in various areas both in vitro and in vivo (animal models and humans). However, although no adverse events were reported after MenSC treatment in humans and therapeutic effects were observed in a study on multiple sclerosis 136 and AS 61, more studies are required to evaluate any challenges prior to MenSC application in a routine setting.
There are also some limitations to use of MenSC that need to be acknowledged. MenSC have high rate of proliferation, however, time is required for the cells to multiply and achieve quantities which are sufficient for therapeutic applications. Collection of MenSC can be obtained only from pre-menopausal women; however, also males and post-menopausal women are prone to diseases such as stroke and neurodegenerative disorders. This problem could be solved by educating females on the potential therapeutic applications of menstrual cells and by offering harvesting and cryopreservation to pre-menopausal women for future autologous use. Allogenic menstrual blood can be used to treat males and even post-menopausal women 43.
Although MenSC exhibit some limitations, they also exhibit various advantages which may outweigh these limitations. They have shown a potential in future therapy in various areas. It is important that further studies, especially in humans, do not just evaluate the long-term safety of MenSC but also the effects of MenSC on various diseases. Moreover, establishing a protocol that maximizes the therapeutic potential of MenSC with limited adverse reactions is also crucial 137. It is important that a standardized protocol is devised for MenSC according to the diseases and also to the different age groups that might receive this treatment. Various routes of MenSC administration have been investigated and there is the need for studies which focus on the best method to transplant MenSC to achieve maximal therapeutic benefit according to the disorder. MenSC have shown therapeutic effects in various diseases, however, the underlying mechanisms and signal pathways are still unknown 39,138. Although more work is required on MenSC, they have shown multi-functional roles in the treatment of various diseases in preclinical research which paves the way for further development of MenSC therapy in clinical and regenerative medicine 39.
Conclusion :
MenSC have shown a very promising therapeutic effect in various diseases. Studies conducted in humans with H7N9, COVID-19, CHF, MS and DMD reported positive therapeutic effects without any adverse events. However, more studies are required to evaluate any challenges prior to MenSC application in a routine setting. Further research on MenSCs is required regarding age of donor, transplantation routes, appropriate dose, eligible conditions, long-term monitoring and mechanisms of action. It is important that a standardised protocol is devised for MenSC according to the diseases and to the different age groups that might receive this treatment. No long-term data on MenSC exist and therefore more studies should focus on this aspect to examine their long-term safety and the survival time of MenSC. Although more work is required on MenSC, they have shown multi-functional roles in the treatment of various diseases in preclinical research which paves the way for further development of MenSC therapy in clinical and regenerative medicine. The ease of rapidly obtaining MenSC in large amounts makes them unique, thus sperheading their application in clinical practice.
Acknowledgement :
The authors declare no conflict of interest.

Appendix 1. Medline (ovid) (from 2007 to august 2020, week 4)
|

Appendix 2. Embase (from 2007 to august 2020, week 4)
|
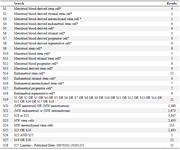
Appendix 3. Cochrane central register of controlled trials (EBSCO) (from 2007 to august 2020, week 4)
|
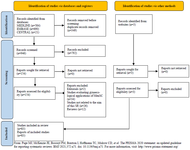
Figure 1. PRISMA flow diagram study identification
|
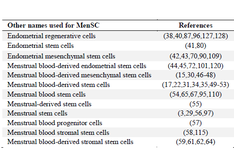
Table 1. Nomenclatures used for MenSC (39)
|

Table 2. Preclinical animal studies involving menstrual-derived stem cells in non-gynaecological diseases
|

Contd. Table 2. Preclinical animal studies involving menstrual-derived stem cells in non-gynaecological diseases
ALI: Acute Lung Injury, ALT: Alanine Aminotransferase, AST: Aspartate Aminotransferase, BDNF: Brain-Derived Neurotropic Factor, BLM: Bleomycin, DSS: Dextran Sulfate Sodium, FiO2: Fraction of Inspired Oxygen, GSK-3β: Glycogen Synthase Kinase 3 beta, HGF: Hepatocyte Growth Factor, IC: Intracranial, ICAM-1: Intracellular Adhesion Molecule 1, IL: Interleukin, IV: Intravenous, MenSC: Menstrual-derived Stem Cells, MI: Myocardial Infarction, MMP: Matrix Metalloproteinase, mtDNA: mitochondrial Deoxyribonucleic acid, paO2: Arterial Pressure of Oxygen, ROS: Reactive Oxygen Species, SCC: Squamous Cell Carcinoma, SCI-spinal Cord Injury, sO2%: Oxygen Saturation, TNF: Tumour Necrosis Factor, TRAIL: Tumour Necrosis Factor-Related Apoptosis-Inducing Ligand, VEGF: Vascular Endothelial Growth Factor.
|
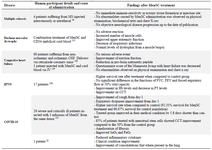
Table 3. Clinical studies involving potential applications of menstrual derived stem cells in humans
CCT: Chest Computer Tomography, CD34: Cluster of Differentiation 34, CHF: Congestive Heart Failure, FEV: Forced Expiration Volume, FVC: Forced Vital Capacity, Hb: Haemoglobin, IV: Intravenous, MS: Multiple Sclerosis; PT: Prothrombin Time.
|

Table 4. Cellular differentiation ability of menstrual-derived stem cells
bFGF: basic Fibroblast Growth Factor, CK-18: Cytokeratin 18, DMEM-HG: Dulbecco’s Modified Eagle’s High Glucose, FBS: Foetal Bovine Serum, FGF2: Fibroblast Growth Factor-2, GABBR: Gamma-aminobutyric Acid type B Receptor, GFAP: Glial Fibrillary Acid Protein, HGF: Hepatocyte Growth Factor, IGF-1: Insulin-like Growth Factor 1, ITS+1: Insulin Transferrin Selenium pre-mix, LEPR: Leptin Receptor, LPL: Lipoprotein Lipase, MAP2: Microtubule-Associated Protein 2, MenSC: Menstrual Derived Stem Cells, OSM; Oncostatin M, OSTF1: Osteoclast-Stimulating Factor-1, PPAR-γ: Peroxisome Proliferator-Activated Receptor Gamma, TGF-β1: Transforming Growth Factor beta 1, TUBB3: Tubulin beta 3 Class III.
|
|