Human Tissue Plasminogen Activator Expression in Escherichia coli using Cytoplasmic and Periplasmic Cumulative Power
-
Majidzadeh, Keivan
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Iranian Center for Breast Cancer (ICBC), ACECR , Tehran, Iran
-
Mahboudi, Fereidoun
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Hemayatkar, Mahdi
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Davami, Fatemeh
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Barkhordary, Farzaneh
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Adeli, Ahmad
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
Davoudi, Noushin
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
-
 Khalaj, Vahid
Vahid Khalaj, Ph.D., Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66480780 Fax: +98 21 66480780 E-mail:khalajs@pasteur.ac.ir
Khalaj, Vahid
Vahid Khalaj, Ph.D., Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66480780 Fax: +98 21 66480780 E-mail:khalajs@pasteur.ac.ir
-
Biotechnology Department, Biotechnology Research Center, Pasteur Institute of Iran , Tehran, Iran
Abstract: Tissue plasminogen activator (tPA) is a serine protease, which is composed of five distinct structural domains with 17 disulfide bonds, representing a model of high-disulfide proteins in human body. One of the most important limitations for high yield heterologous protein production in Escherichia coli
(E. coli) is the expression of complex proteins with multiple disulfide bridges. In this study the combination of two distinct strategies, manipulated cytoplasm and native periplasm, was applied to produce the functional full length tPA enzyme in E. coli. Using a PelB signal peptide sequence at 5' site of tPA gene, the expression cassette was prepared and subsequently was transformed into a strain with manipulated oxidizing cytoplasm. Then the induction was made to express the protein of interest. The SDS-PAGE analysis and gelatin hydrolysis confirmed the successful expression of functional tPA. The results of this study showed that complex proteins can be produced in E. coli using the cumulative power of both cytoplasm and periplasm.
Introduction :
Tissue plasminogen activator (tPA) is a ~64 kDa serine protease that converts the plasminogen to plasmin, a serine protease of broad specificity that degrades the fibrin network in thrombi. tPA is composed of five distinct structural domains; a finger region, an epidermal growth factor-like sub-domain, two kringle domains, and finally, the catalytic domain. It is a 527-amino acid protein, with 35 cysteine residues that participate in formation of 17 disulfide bonds, representing
a model of high-disulfide proteins in human body (1,2).
Prokaryotic systems such as E.coli have been the most widely used systems for the recombinant protein production. This is mainly due to genetic simplicity, fast growth rate, high cell density production and the availability of an increasingly large number of vectors and host strains (3 - 5). One of the most important limitations for high yield heterologous protein production in E. coli is the expression of complex proteins with multiple disulfide bridges. Among the several factors affecting the efficiency of such complex proteins production (6 - 9), the reducing environment of cytoplasm seems to play a key role in improper folding of such high disulfide bonded proteins. The periplasm of E.coli is more oxidizing environment and in wild type bacteria is more suitable than cytoplasm for proper folding (3).
There are two common in vivo approaches to improve the conformation of complex proteins in E. coli. The first is manipulating the condition of cytoplasm by converting its reducing nature into an oxidizing environment. In this approach the gene of interest, without the signal peptide encoding sequence is transformed into the engineered E. coli strain (10). As a result, the recombinant protein maintains in oxidizing environment of cytoplasm and the proper conformation of the protein forms.
In the second approach, the secretion of the protein into the less reducing environment of periplasm is the main idea. To achieve this goal, the gene of interest containing a suitable signal peptide is introduced to the bacterial host and the signal peptide directs the protein into the periplasm.
The aim of this study was to investigate the potential of using a signal peptide for production of a highly disulfide bonded protein in an E. coli strain with engineered cytoplasm. So, for the first time, we have used both approaches by a simple method that can be improved in future studies. Using a signal peptide sequence at 5' site of tPA gene, the expression cassette was prepared and subsequently was transformed into a strain with manipulated oxidizing cytoplasm. In this way the protein of interest is produced in oxidizing environment of cytoplasm and the disulfide bonds would be formed to some extent. Then signal peptide transfers the produced protein into the periplasm where a greater amount of the disulfide bonds would be formed in less reducing environment of periplasm.
In this introductory study, tPA was cloned and expressed in an E.coli strain with oxidizing specialty. The expression and function of tPA were assayed by SDS-PAGE and Gelatin Hydrolysis test.
Materials and Methods :
Strains, plasmids and culture media
Escherichia coli strain Top10 F' was used as the host for recombinant plasmid. Origami B (DE3) was used as expression host. pTZ57R (Fermentas, Vinius, Lithuania) as T/A cloning vector and pET22b as expression vector were used in experiments. pET22b is a bacterial vector with the size of 5.5 kb and it contains PelB sequence for periplasmic localization. LB agar and Broth were used for culturing the strains.
PCR amplification and cloning of human tPA gene
Genomic DNA of CHO 1-15 cell line (ATCC- CRL 9606) transfected by full length cDNA of human tPA (GenBank accession number 101047), was used as template for PCR amplification. FortPA (5’-AACCATGG ATGCAATGAAGAGAGGGCTC -3') containing NotI restriction site and RevtPA (5'- GCGGCCGCTCACGGTCGCATGTTG -3') containing NcoI restriction site were used as forward and reverse primers, respectively. A high fidelity PCR reaction was set with following thermal cycles: 2 min at 95 °C for one cycle, and 30 cycles of 1 min at 95 °C, 45 sec at 68 °C, 2 min at 72 °C, and a final extension cycle of 10 min at 72 °C.
The resulting PCR product was tailed by an oligo A at 3' side and subsequently was cloned into the pTZ57R vector (Fermentas, Vinius, Lithuania), generating pTZ-tPA. Restriction mapping and bidirectional sequencing of cloned fragment was performed to confirm the construct. To prepare the final construct, tPA cloned fragment was cut from pTZ-tPA vector using NotI and NcoI enzymes and subsequently cloned into pET22b. The final construct was called pET/ tPA and confirmed by restriction analysis and sequencing.
Transformation of origami B (DE3) cells and expression of recombinant tPA
Competent cell preparation and transformation was done using standard Calcium chloride method. The transformed cells were cultured in LB Agar containing Tetracycline. For expression experiments, transformants were cultured in 5 ml LB broth and the induction was carried out by adding IPTG 1 M at the optical density of 0.3 - 0.5 in 600 nm.
SDS-PAGE analysis and zymography test
Cell lysis and SDS-PAGE analysis on 12% polyacrylamide gels were performed according to standard methods (11).
Zymography test was performed as described before (12,13). Briefly, the protein samples were separated on a 11% non-reducing SDS-poly acrylamide gel containing appropriate amount of Plasminogen (Chromogenix, Italy) and Gelatin (Sigma, USA). After complete separation, the gel was soaked in 2.5% (w/v) Triton X-100 at room temperature for 1 hr to remove SDS and then incubated in 0.1 M glycine/ NaOH (pH=8.3) for 5 hr at 37 °C. The gel (zymogram) was subsequently stained with Coomassie Brilliant Blue and the areas of digestion appeared as clear bands against a darkly stained background where the substrate has been degraded by the enzyme.
Result :
Amplification and cloning of tPA cDNA
Human tPA cDNA was amplified by specific primers (Figure 1) and the resulting fragment was cloned into pTZ57R; successfully. Figure 2, lane 1 shows the result of restriction digestion of pTZ/ tPA by NotI and NcoI enzymes which created two fragments; the backbone of cloning vector (2.9 kb) and tPA fragment.
The size of expression construct (pET/tPA) was 7.2 kb and the restriction map of this construct using NotI and NcoI enzymes showed two fragments with expected sizes at 5.5 kb and 1.7 kb, confirming a successful sub-cloning of tPA gene into pET22b expression vector (Figure 3). Final sequencing of the construct confirmed the presence and correct orientation of insert inside the vector (data not shown).
Expression of recombinant tPA
Expression of recombinant tPA was induced by addition of IPTG as described above. A positive transformant was selected and was grown in LB medium until the cell optical density (OD, 600 nm) reached to 0.3-0.5.
Following induction, four hour- samples were taken and the lysates of induced and non-induced cells were compared in a standard SDS-Polyacrylamide gel (Figure 4). In figure 4, lanes 2 and 4 represent the expressed band of tPA in cell lysate of recombinant Origami B (DE3) after four hr of induction (arrows).The band related to t-PA protein expressed by Origami B (DE3) transformant is shown by the arrow. Lanes 1 and 3 show the protein background of expression host before induction. The lack of expression in columns 1 and 3 shows a tight regulation of expression in pET22b vector. Lane 5 represents the molecular weight marker bands.
Gelatin hydrolysis test
In Zymography test, the plasminogen and gelatin were co-polymerized and immobilized with a non-reducing polyacrylamide gel. The cell lysate of transformed expression host before and after induction were applied to evaluate the activity and conformation of recombinant tPA (Figure 5). The transparent regions on the gel which was observed only in transformed host (Lane 7 of Figure 5), indicated that the plasminogen is digested by serine-protease activity of t-PA and derived plasmin resulted in gelatin hydrolysis. Commercial t-PA (Actylase) was used as positive control (Lanes 6 of Figure 5). Cell lysate of transformed expression host before induction was used as negative control (Lanes 1-5 of Figure 5).
Discussion :
Tissue plasminogen activator is an important enzyme for biotechnology industry due to its extended application in medicine (14 - 16). It is one of the most complex proteins in human body and currently is produced in CHO which is a mammalian host and so the production cost is very high (1). Attempts to produce active tPA in Saccharomyces cerevisiae or in insect cells have been frustrated by problems like hyperglycosylation, poor export, and improper folding (17 - 19).
Conclusion :
Recombinant expression of a highly disulfide bonded protein, human t-PA, in E. coli was investigated in this study. As the functionality of t-PA is highly dependent on its proper folding and disulfide bond formation, it can be concluded that the application of two distinct strategies, manipulated cytoplasm and native periplasm would be an effective approach in recombinant production of complex proteins.
Acknowledgement :
The authors would like to thank Dr Behrouz Vaziri for his valuable comments. This work is financially supported by Pasteur Institute of Iran.
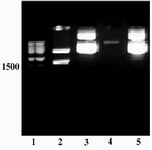
Figure 1. Amplification of tPA cDNA using genomic DNA of CHO 1-15 cell line. A specific band of ~1.7 kb was amplified (lane 1). Lane 2: size marker
|
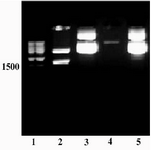
Figure 2. Restriction analysis of pTZ/tPA construct. Lane 1) Size marker. Lane 2) Fragments created by NcoI/NotI digestion of the construct; The backbone plasmid (2.9 kb) and tPA fragment (1.7 kb) are present. Lane 4) NcoI/NotI linearized pET22b plasmid. Lanes 3 and 5 represent undigested plasmids
|
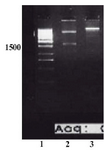
Figure 3. Restriction analysis of pET/tPA construct. Lane 2 shows two fragments created by NcoI/NotI digestion. The back bone plasmid, pET22b vector (5.5 kb), and tPA fragment (1.7 kb) are present. Lane 3 shows the undigested plasmid. Lane 1: Size marker
|
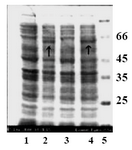
Figure 4. SDS-PAGE analysis of a recombinant clone producing tPA. Lanes 1 and 3 show the protein back ground of expression host before induction. Lanes 2 and 4 represent the expressed band of t-PA in cell lysate of recombinant Origami B (DE3) after four hours of induction. Arrows indicate the bands related to recombinant tPA
|
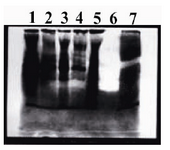
Figure 5. The cell lysate of transformed expression host before and after induction were applied to evaluate the activity and conformation of recombinant t-PA. Lane 7 shows the transparent band of cell lysate after induction, which indicated that the plasminogen is digested by serine-protease activity of t-PA and derived plasmin resulted in gelatin hydrolysis. Commercial t-PA (Actylase) was used as positive control (Lanes 6). Cell lysate of transformed expression host before induction (Lane 5) and negative colonies (Lanes1-4) after induction were used as negative controls
|
|