Downregulation of MMP2 and Bcl-2 in Adipose Derived Stem Cells (ASCs) following Transfection with IP-10 Gene
-
Razmkhah, Mahboobeh
-
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Jaberipour, Mansooreh
-
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
 Ghaderi, Abbas
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, Tel: +98 711 230 4952; Email: ghaderia@sums.ac.ir
Ghaderi, Abbas
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran, Tel: +98 711 230 4952; Email: ghaderia@sums.ac.ir
-
Shiraz Institute for Cancer Research, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
Department of Immunology, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
Abstract: Background: Mesenchymal Stem Cells (MSCs) are recently introduced as novel immunological gene carriers for treatment of cancer. It is believed that balance between the expression of angiogenic and anti-angiogenic factors, such as SDF-1 and IP-10, may regulate neovascularization within the tumor. Methods: In this study, we compared the expression of important tumor promoting mediators in IP-10-transfected Adipose Derived Stem Cells (ASCs) to those transfected with SDF-1. ASCs were isolated from adipose tissue of a normal subject undergoing cosmetic mamoplasty surgery using collagenase. ASCs were transfected with IP-10 or SDF-1 propagated plasmids by electroporation method and Lipofectamin 2000. Expressions of SDF-1, CXCR4, IP-10, Bcl-2, MMP2, IL-10, IGF-1, and VEGF were detected in transfected ASCs using quantitative Real-Time Polymerase Chain Reaction (qRT-PCR). Results: Results showed that the expressions of SDF-1, CXCR4, Bcl-2, MMP2, IL-10, IGF-1, and VEGF were upregulated in SDF-1-transfected ASCs. In contrast, Bcl-2 and MMP2 transcripts showed 45×103 and 10 fold lower expression in ASCs transfected with IP-10 compared to non-transfected cells. Conclusion: Anti-angiogenic chemokines such as IP-10 may modulate tumor promoting properties of ASCs and would be introduced as novel candidates for tumor immunotherapy; however, further studies are needed to be conducted.
Introduction :
Due to their poor immunogenicity, MSCs are introduced as appropriate candidates for clinical applications. Since MSCs have tumor tropism, they may be used as vehicles for tumor-specific delivery of anti-tumor molecules, such as immune related genes 1. Application of adenoviral infected MSCs with IFN-β gene which could successfully inhibit the tumor growth and angiogenesis in melanoma, breast cancer, and glioma models have been reported previously 1. Similarly, genes of inflammatory cytokines, such as IL-2 and IL-12, are known for activating anti-tumor immune responses when they were used with MSCs as gene carrires 2.
Other candidate genes for anti-tumor immunotherapy are anti-angiogenic chemokines among which, interferon-inducible protein-10 (IP-10 or CXCL10) has recently been introduced. IP-10 is secreted by several cell types such as monocytes, endothelial cells and fibroblasts in response to IFN-γ 3. This chemokine is a chemoattractant for monocytes, macrophages, T cells, NK cells and dendritic cells. Also, it has the ability to promote T cell adhesion to endothelial cells, antitumor activity, and to inhibit bone marrow colony formation and angiogenesis 4,5.
Melanoma tumor cells transfected with IP-10 gene showed outstanding reduction in tumor growth and promotion in comparison with vector-transfected tumor cells 6. It has recently been described that combination of anti-angiogenic therapy with chemotherapy has much better therapeautic effects 7. IP-10 expressing plasmid DNA and gemcitabine injection into tumor-bearing mice led to the inhibition of tumor growth through recruiting lymphocytes, induction of tumor cell apoptosis, and longer survival time in tumor-bearing mice 7. Yang et al have shown that IP-10 is involved in regulating the proliferation, survival, and activation of tumor-specific T cells other than mediating anti-angiogenic effects in tumor microenvironment 8. Thus, in vivo targeted expression of IP-10 is considered as a potentially useful approach for immunotherapy of cancer 9.
In contrast to IP-10, SDF-1 axis contributes to angiogenesis through induction of proangiogenic factors such as IL-8 and VEGF 10. SDF-1 has multiple physiological functions that include homing of hematopoietic stem cells to bone marrow during fetal life and marrow transplantation 11, contribution to the homing of peripheral blood lymphocytes in peripheral lymphoid tissues such as lymph nodes and regulation of pre-B cell trafficking within bone marrow microenvironment as a chemoattractant factor 12. It has also pivotal roles in the development and function of nervous system 13,14.
About the role of SDF-1 in cancer promotion, it has been reported that myofibroblasts derived-SDF-1 increases the growth of breast cancer cells through angiogenesis and tumor cells proliferation 15. Therefore, antibodies against SDF-1 receptor, CXCR4, have the ability to inhibit SDF-1-CXCR4 interaction and prevent tumor growth and angiogenesis 16. Fernandis AZ et al reported that stimulation of MDA-MB-231 breast carcinoma cells by SDF-1 leads to release of MMP2 and MMP9 and to increase cellular motility and migration 17. In contrast, the epigenetic silencing of SDF-1 increases the metastasis of breast cancer cells and its forced expression decreases the number of metastases, in vivo 18.
In addition to the application of MSCs as novel immunological gene carriers, tumor promoting activity of these cells 19,20 is a critical concern which can be modulated through transferring the immune related genes to these cells. For this purpose, present study aims to investigate the effects of IP-10 (as a potent anti-angiogenic factor) on the expression profile of tumor promoting molecules in adipose derived stem cells (ASCs). Results are compared to ASCs transfected with SDF-1 gene, as an angiogenic factor.
Among the different sources for isolating mesenchymal cells, bone marrow has been considered as the major source. However, MSCs has recently been found in other tissues such as adipose tissue 21. Although, MSCs isolated from both adipose tissue and bone marrow expanded easily in vitro, ASCs showed a higher proliferation rate and longer survival time in culture than bone marrow stem cells 22. Adipose tissues are most safe and easy to harvest with low risk and are highly abundant in the human body 23. Also, it is of particular interest due to the increased incidence of obesity and liposuction surgeries that are performed each year 21. Considering the advantages of ASCs, here we used the adipose tissue as a source for extracting MSCs.
Materials and Methods :
Isolation and characterization of ASCs: ASCs were extracted from breast adipose tissues of a healthy donor with cosmetic mammoplasty surgery after obtaining written informed consent. Fragments of the adipose tissue were washed with Phosphate Buffered Saline (PBS), minced in small pieces, and digested with 0.2% collagenase type I (GIBCO, USA) at 37C. The resulted soup was centrifuged at 400 g for 10 min and then the pellet was incubated with RBC lysis buffer and centrifuged for 10 min at 300 g. Stromal Vascular Fraction (SVF) was isolated and cultured in DMEM culture medium (GIBCO) containing 10% fetal bovine serum (GIBCO) using Ficoll hypaque (Biosera) gradiant centrifugation. Non-adherent cells were discarded after 24 hr and the adherent cells were cultured by changing the medium every 4 days and harvested in passages 3-5.
As we showed in our previous investigations, for characterization ASCs were stained with various combinations of phycoerythrin (PE)-conjugated mouse anti-human CD44, CD105, and CD166 (all from BD Biosciences, USA) and fluorescein isothiocyanate (FITC)-conjugated mouse anti-human CD14, CD34, and CD45 (all from BD Biosciences, USA). Isotype-matched irrelevant monoclonal antibodies (BD-pharmingen, USA) were used as negative controls. Flow cytometric analysis was performed on a FACS Calibur machine (BD Biosciences, USA) using the Cell quest as data acquisition software 19,24,25.
To further characterize the growing cells, they were differentiated into chondrocytes and hepatocytes as described before 19,24,25.
Preparation of competent cells, transformation, and plasmid DNA propagation: DH5-α and XL2 competent Escherichia coli (E. coli) strains were prepared for transformation based on the Hanahan method (Hanahan et al, 1983). Then, the competent cells were transformed with 1 μl of appropriate plasmids (OriGene, USA) using heat shock method. E. coli XL2 and DH5-α bacterial cells were transformed with pCMV6-XL5 (contained SDF-1 or IP-10 genes) and pEGFP-N1 (contained GFP gene) plasmids, respectively. Plasmid DNAs were extracted and propagated using appropriate S.N.A.P. Miniprep and then Midiprep kits according to the manuscripts guidlines (Invitrogene, USA). Then, the plasmid DNAs were validated with restriction enzyme digestion based on the genetic map of plasmids using Not1 (Fermentas, Canada) for pCMV6-XL5 plasmid and Not1 and Hind III (Fermentas, Canada) for pEGFP-N1 plasmid.
Transfection of ASCs using electroporation method: One-two days prior to electroporation, ASCs were transferred to the 75 cm2 culture flasks with fresh 10% DMEM medium in order to have 50-70% confluent cells on the day of the experiment. ASCs were detached using trypsin–EDTA (Biosera, USA), washed with PBS, gently pipetted several times using insulin syringe in order to have single cells, and counted using a hemocytometer. The cells were then washed with DMEM medium without serum and approximately 2×106 cells were transferred into an electroporation cuvette. Afterward, the cells were incubated with 400 μl hypo-osmolar buffer (Invitrogene USA) for 15-20 min at room temperature. About 20 μg plasmid DNA was added to the cells and electroporated with the square wave program with three 350 V pulses using a BioRad Gene Pulser. Both pulse length and pulse intervals were 10 (different electroporation setups are shown in Supplementary Table 1). Electeroporated ASCs were then incubated on ice for 1 min and immediately transferred to the culture flask containing 10% fresh DMEM medium. First, the expression of GFP protein was assessed using flow cytometry at 24, 48, 72, and 96 hr after the electroporation. Untransfected and GFP transfected ASCs were used as the control groups. Expressions of GFP, IP-10 and SDF-1 were evaluated using either flow cytometry or quantitative real time polymerase chain reaction (qRT-PCR). Three biological repeats were conducted for each test.
Transfection of ASCs using lipofectamin 2000: Another method used for transfection of ASC was lipofectamine transfection using Lipofectamine 2000 (Invitrogene, USA) based on the manufacturer's recommendation. For optimizing the procedure, a specific concentration of DNA should be mixed with the appropriate amount of lipofectamine 2000. The ratios of Lipofectamine to GFP plasmid DNA concentration (μl/μg) used for setting up the test were selected from the recommended amounts in the manuscript and changed to get the best result. These ratios were: 0.4/0.8, 0.8/0.8, 1.2/0.8, 1.6/0.8, 2/0.8, 2.4/0.8, 4/0.8, 4/3.2, 2/4.8, and 5/2.4. Expressions of GFP, IP-10 and SDF-1 were evaluated using either flow cytometry or quantitative real time polymerase chain reaction (qRT-PCR).
RNA isolation, cDNA synthesis and qRT-PCR
Total RNA was extracted from ASCs using TRizol reagent (Invitrogen, Paisley, UK) and the phenol chloroform method. Before cDNA synthesis, the extracted RNAs were treated with DNase I (Invitrogen-Gibco, Paisley, UK) to avoid DNA contamination. Furthermore, cDNA was synthesized from 5 μg of total RNA using the RevertAid First Strand cDNA Synthesis Kit (Fermentas, Vilnius, Lithuania).
Expressions of Bcl-2, VEGF, MMP2, IGF-1, IL-10, CXCR4, SDF-1, and IP-10 gene transcripts were determined 1, 2, 5, and 7 days after electroporation through the qRT-PCR method using SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA). Each PCR reaction was carried out in duplicate in a final volume of 25 μl containing, 12.5 μl of 2x SYBR Green Master Mix, 0.2 μl of each 10 pmol forward and reverse primers which were designed in Primer Blast software (Table 1), 2 μl cDNA and 10.1 μl DEPC treated water. PCR amplification was done in 40 cycles using the following program: 95ºC for 10 min, 95ºC for 15 s, 56ºC for 30 s, and 60ºC for 34 s. Finally, the data were compared to those from beta actin housekeeping gene.
Statistical analysis: Expression of the molecules in transfected ASCs was reported using 2-∆∆CT method compared to non-transfected ASCs. Besides, the average of CTs was used for 2-∆∆CT estimation (Critical factors for Successful Real-Time PCR, www.qiagen.com, 2006).
Results :
Phenotyping and differentiation properties of ASCs: ASCs were observed as homogenous spindle-shaped cell population. Flow cytometry analysis revealed that the cultured ASCs were approximately 98% positive for the expressions of CD44, CD105, and CD166, while they had no significant expression of hematopoetic specific markers; i.e., CD14, CD34, and CD45 (Supplementary Figure 1) 19. The hepatogenic and chondrogenic differentiations showed morphologic changes of ASCs into the polygonal/flattened and rounder/cuboidal shaped cells, respectively. Expression of several hepatogenic and chondrogenic specific genes could also reasonably verify the isolated cells as ASCs (Supplementary Figures 2 and 3) 19,24,25.
GFP expression in ASCs using electeroporation or lipofectamine 2000: ASCs were transfected with IP-10 and SDF-1 plasmids in order to show the effects of IP-10 or SDF-1 overexpression on the expressions of tumor promoting factors. Electroporation and transfection with Lipofectamine 2000 were set up using GFP plasmid. The best result was gained by the electroporation method with 10% efficiency (Figure 1) after 72 hr. In addition, the best electroporation program was a square wave program with three 350 V pulses and both pulse length and pulse intervals were equal to 10 (program 13, Supplementary Table 1).
The best efficiency with Lipofectamin 2000 was 1.3% which was gained by the Lipofectamine to DNA concentration ratio of 4/0.8 (μl/μg).
As the efficiency of the electroporation method was higher than using Lipofectamin 2000, expressions of Bcl-2, VEGF, MMP2, IGF-1, IL-10, and CXCR4 were assessed in the ASCs transfected by the electroporation method.
mRNA expressions of IP-10 and SDF-1 in IP-10, SDF-1, and GFP transfected ASCs: IP-10 had 6×107 fold more mRNA expression at day 7 in the IP-10 transfected cells compared to the non-transfected ASCs. Nonetheless, no change was observed in the expression of IP-10 in GFP transfected ASCs. SDF-1 mRNA had 14×104 fold more expression in the ASCs transfected with SDF-1 compared to the non-transfected ASCs at day 5. However, no obvious change in the expression of SDF-1 was observed in GFP transfected ASCs (Figure 2).
Expressions of Bcl-2, MMP2 and VEGF in ASCs after transfecting with IP-10 plasmid: As shown in figure 3, expressions of Bcl-2 and MMP2 transcripts decreased at day 7 after transfection of ASCs with IP-10. Bcl-2 and MMP2 transcripts showed 45×103 and 10 fold lower expression compared to the untreated cells at this day. The mRNA expression of VEGF increased after transfection with IP-10, and its expression was almost 5 times more than the control group.
Expressions of Bcl-2, MMP2, VEGF, CXCR4, IGF-1, and IL-10 in ASCs after transfecting with SDF-1: mRNA expression of Bcl-2 increased to 2.5×103 fold more than untreated cells at day 5 after transfection of ASCs with SDF-1. Similarly, expressions of MMP2 and VEGF transcripts showed about 330 and 2×103 fold increase in comparison to the non-transfected ASCs, respectively (Figure 4).
SDF-1 caused CXCR4 mRNA augmentation to approximately 3×103 fold in comparison to the non-transfected ASCs. In addition, SDF-1 plasmid led to IL-10 and IGF-1 mRNA upregulation to 370 and 1×105-fold in ASCs at day 5 compared to the non-transfected ASCs (Figure 5).
Discussion :
It has been evidenced that MSCs inhibit the proliferation and responses of both primary and activated T cells through Th2 type cytokine production 26. They also contribute to induction of tumor infiltrating T lymphocytes toward the regulatory T cells 24,27. MSCs’ derived soluble factors, such as indolamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2), Transforming Growth Factor-β1 (TGFβ1), Hepatocyte Growth Factor (HGF) 28-30, Leukemia Inhibitory Factor (LIF) 31, and HLA-G5 32 are considered as known immuno-modulatory mechanisms of these cells. MSCs have the ability to transdifferentiate into Cancer Associated Fibroblasts (CAFs) and express the main molecular markers of CAFs, including SDF-1, under the influence of TGF-β 33,34. Interestingly, MSCs are one of the main sources of TGF-β in the tumor microenvironment which is named as the master regulator of metastasis through EMT mechanism 20.
In contrast to the tumor promoting effects of MSCs, they are known as one of the best gene carriers for the delivery of immunological genes to the tumor microenvironment 35 because of their ability to access the inflammatory sites after i.v. administration 36. Based on these reports, we hypothesized that transfection of ASCs with an angistatic gene, such as IP-10, may modulate the functional properties of ASCs and present them as better gene carriers for cancer gene therapy. Thus, we firstly aimed to increase the efficiency of our transfection methods, including electroporation and lipofectamin. The best result of transfection with IP-10 or SDF-1 plasmids was obtained by electroporation because both genes were expressed in large amounts in our transfected ASCs by this method. Then we assessed the effect of IP-10 and SDF-1 overexpression on the expression of tumor promoting cytokines. As mentioned in result section, transfection of ASCs by IP-10 led to reduced amounts of Bcl-2 and MMP2 transcripts in ASCs.
It has been demonstrated that IP-10 may act as similar as IL-12, regarding its antitumor effects 37. Luster et al genetically engineered tumor cells to secrete high levels of murine IP-10 and demonstrated that IP-10 has a strong antitumor effect in vivo that is mediated through the recruitment of lymphocytes, neutrophils, and monocytes 38. It has also been shown that the overexpression of MIG, IP-10, and CXCL11 in the local tumor inhibits tumor growth and metastasis through the angiostatic effect or activation of T lymphocytes or NK cells 39.
These findings and our results which showed downregulation of MMP2 and Bcl-2 in IP-10 transfected ASCs explain the importance of IP-10 in tumor immunotherapy. Additionally, combination therapy with IP-10 and other antitumor agents, such as IL-12, may have more significant tumor regressive effects compared to IP-10 or IL-12 alone 37. However, upregulation of VEGF after using IP-10 in ASCs is not consistent with other investigations which showed the inhibiting effects of IP-10 on the endothelial cells 40,41. Thus, the present results may impose us to do further studies for better elucidation.
Controversial results have been obtained after transfecting different types of cells by SDF-1 gene. Evidences showed that saturation of CXCR4 through autocrine SDF-1 production reduces chemotaxis to the target organs which were rich in SDF-1 18. Interestingly, adenoviral gene delivering of SDF-1 to tumors might inhibit the growth of preexisting tumors through attraction and accumulation of dendritic cells to the tumor site 42. SDF-1 has been introduced as an effective adjuvant to augment anti-tumor responses through recruitment of CXCR4 expressing T cells 43. In contrast, there are several reports showing the tumor promoting effects of SDF-1 for instance in angiogenesis of the tumor 15,16. Treatment of human colon epithelial cells with SDF-1 can induce the production of IL-8 as well as GROα 44. Stimulation with SDF-1 led to secretion of a variety of MMPs and to cellular motility 17,45. Our results confirmed the tumor promoting effects of SDF-1 because CXCR4, Bcl-2, MMP2, VEGF, IL-10, and IGF-1 had more expressions after transfecting with SDF-1 gene. Thus, we conclude that the production of SDF-1 in tumor microenvironment may contribute to the tumor growth and metastasis.
Conclusion :
Overall, it is concluded that adipose tissue is a suitable source for extracting stem cells and applying them as immunological gene carriers to the tumor site. In addition, among the candidate genes IP-10 may be introduced as an anti-tumor therapeutic agent to suppress tumor progression and metastasis. However, more investigations in experimental models are required.
Acknowledgement :
This work was financially supported by Shiraz University of Medical Sciences [Grant No. 87-4218] and Shiraz Institute for Cancer Research [ICR-100-504].
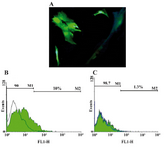
Figure 1. A) Transfection of ASCs with GFP plasmid; B) ASCs were firstly transfected with pEGFP-N1 plasmid and the best efficiency was gained with electroporation method; C) compared to Lipofectamine 2000 (10% vs 1.3%, respectively)
|
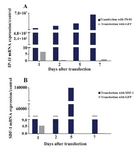
Figure 2. A) mRNA expressions of IP-10; B) and SDF-1; after transfecting ASCs with plasmids DNA encoding GFP, IP-10 or SDF-1
|
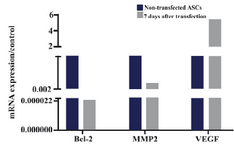
Figure 3. 2-ΔΔCt of Bcl-2, MMP2 and VEGF in ASCs, 7 days after transfecting with IP-10 plasmid and in non-transfected cells
|
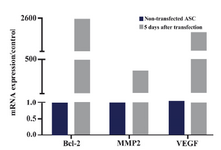
Figure 4. Bcl-2, MMP2 and VEGF mRNA expressions in ASCs 5 days after transfecting with SDF1 plasmid and in non-transfected cells. The data were shown as 2-ΔΔCt
|
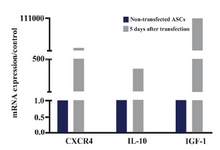
Figure 5. IL-10, CXCR4 and IGF-1 mRNA expressions in ASCs 5 days after transfecting with SDF1 plasmid and in non-transfected cells. The data were shown as 2-ΔΔCt
|

Supplementary figure 1. The expressions of ASCs’ specific markers were analyzed by flow cytometry and results are representative of on experiment. Filled histograms represent the specific markers (CDs) and unfilled histograms were isotype control
|
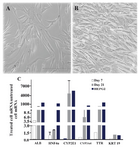
Supplementary figure 2. Differentiation of ASCs to hepatocytes; A) Adipose derived stem cells were observed as spindle shaped cells in culture before differentiation; B) ASCs after differentiation to hepatocytes appeared as the polygonal-flattened cells; C) Expressions of different hepatic related genes such as albumin (ALB), HNF4α, CYP2E1, CYP3A4, transthyretin (TTR) and (cytokeratin) KRT19 in ASCs on days 7 and 21 after treatment for hepatogenic differentiation. Data are shown as mean ± SEM of 2-∆∆Ct
|
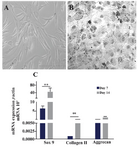
Supplementary figure 3. Differentiation of ASCs to chondrocytes. A) Adipose derived stem cells were observed as spindle shaped cells in culture before differentiation; B) ASCs changed morphologically through chondrogenic differentiation to the cuboidal shapes; C) Expressions of different chondrocyte related genes such as SOX9, collagen type II and aggrecan in ASCs on days 7 and 14 after treatment for chondrogenic differentiation. Data are shown as mean ± SEM of 2-∆∆Ct
|
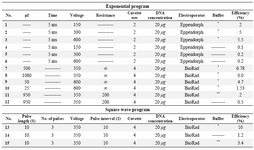
Supplementary table 1. Different electroporation programs. The higher efficiency was gained with method 13. Electroporation was optimized by varying preset electroporation protocols, cell concentrations, and different incubation times before and after electroporation
*BioRad hypoosmolar electroporation buffer.
** Electroporation buffer with the following formula:
10 mM HEPES pH=7.4, 140 mM NaCl, 2.68 mM KCl, 1.7 mM MgCl2, 25 mM glucose pH= 7.4 in 1000 ml ddH2O
|

Table 1. The sequences of primers which were used in qRT-PCR method
|
|