An Anti-TAZ Monoclonal Antibody Recognizing Cell Surface Expressed TAZ Protein in Human Tumor Cells
-
Haji Ghaffari, Mozhan
-
Department of Medical Biotechnology, School of Advanced Science in Medicine, Tehran University of Medical Science, Tehran, Iran
-
Mohammadzadeh, Mahsa
-
Department of Genetics, Islamic Azad University, Tehran Medical Sciences Branch, Tehran, Iran
-
Simonian, Miganoosh
-
Department of Medical Biotechnology, School of Advanced Science in Medicine, Tehran University of Medical Science, Tehran, Iran
-
Hashemi , Mehrdad
-
Department of Genetics, Islamic Azad University, Tehran Medical Sciences Branch, Tehran, Iran
-
Sadeghi, Niloufar
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Negahdari, Babak
-
Department of Medical Biotechnology, School of Advanced Science in Medicine, Tehran University of Medical Science, Tehran, Iran
-
 Mazloomi , Mohammadali
Department of Medical Biotechnology, School of Advanced Science in Medicine, Tehran University of Medical Science, Tehran, Iran, E-mail: ma.mazlomi@gmail.com
Mazloomi , Mohammadali
Department of Medical Biotechnology, School of Advanced Science in Medicine, Tehran University of Medical Science, Tehran, Iran, E-mail: ma.mazlomi@gmail.com
-
 Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 2122432020; Fax: +98 2122432021 E-mail: rabbani@avicenna.ac.ir
Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 2122432020; Fax: +98 2122432021 E-mail: rabbani@avicenna.ac.ir
Abstract: Background: WWTR1 or TAZ is a transcriptional co-activator protein expressed in cytoplasm which functions as the main downstream effector of the Hippo signaling pathway. This pathway is an evolutionally conserved signal cascade, which plays a pivotal role in organ size control and tumorigenesis. Ectopic expression of TAZ has already been observed in many malignancies, while the ectopic localization of TAZ is reported for the first time. The aim of this study was to produce a specific monoclonal antibody (mAb) against a synthetic peptide derived from WWTR1 protein to be used as a research tool in human carcinomas.
Methods: A 21-mer synthetic peptide (derived from human TAZ protein) was used for immunization of BALB/c mice after conjugation with Keyhole Limpet Haemocyanin (KLH) using hybridoma technology. The generated mAb reacted with the immunizing peptide employing ELISA assay. The reactivity of the antibody with native TAZ protein was assessed through Western blot, immunocytochemistry, and flow cytometry using different cancer cell lines.
Results: The produced mAb could recognize the immunizing peptide in ELISA and Kaff was 0.6×10-9 M. The produced anti-TAZ mAb unlike available commercial anti-TAZ antibody, was capable of specifically recognizing cell surface TAZ in human carcinoma cell lines including MCF-7, Raji, and A431 in Western blot, immunocytochemistry, and flow cytometry assays. As expected, no reactivity was observed using normal Peripheral Blood Mononuclear Cell (PBMC) from healthy donors.
Conclusion: Based on the results, TAZ is ectopically expressed on the surface of tumor cell lines which is not the case in normal cells. The generated mAb has a potential to be used as a research tool in studying the expression of TAZ in human carcinomas in different applications.
Introduction :
Cancer is a leading cause of death worldwide, accounting for nearly one in six deaths 1. The burden of cancer is increasing all over the world, and most of the nations will face a major increase in the incidence and mortality of cancer in the next decades mainly in the Low and Middle-Income Countries (LMICs). Worldwide, an estimated 19.3 million new cancer cases and almost 10.0 million cancer deaths occurred in 2020 2. Biomarkers could be used as the tools for various clini-cal settings, including assessing the risk of disease, screening for occult primary cancers, distinguishing benign from malignant findings or one type of cancer from another, determining prognosis and prediction for patients who have malignancies, and checking the grade of the disease; moreover to detect recurrence or determine response or progression to therapy 3. Discovery studies for detecting novel biomarkers and further investigations on identified biomarkers to find their relevance with malignancies could be a valuable effort to improve diagnostic or treatment approaches.
TAZ (transcriptional co-activator with PDZ-binding motif) which is encoded by WWTR1 gene (WW domain-containing transcription regulator1) is one of the main downstream effectors of the Hippo signaling pathway 4. Hippo pathway is a conserved evolutionally signal cascade which plays a vital role in the development of human cancers and in controlling the organ size. TAZ couldn’t act as a transcription factor due to its lack of a DNA binding site however it could participate in gene expression via its intrinsic transactivation domains 5.
TAZ plays its role by regulating of some transcriptional factors that are responsible for the expression of the genes which lead to increased cell proliferation, migration, and differentiation 6. Many studies have shown TAZ serves as a biomarker with prognostic or diagnostic potential and plays an oncogenic role in the development of several cancers such as non-small cell lung cancer 7, breast cancer 8, colorectal cancer 9 and liver cancer 10. In this study, we aimed to develop a mAb against surface TAZ to be applied as a research tool for further investigation of TAZ expression and function in different malignancies.
Materials and Methods :
Immunogen and immunization: A 21 amino acid peptide PESFFKEPDSGSHSRQS-STDS of a region near the N terminal of TAZ protein was designed and synthesized. The immune grade peptide (China Peptides. Co. Ltd) was conjugated with the carrier protein keyhole limpet hemocyanin (KLH, Sigma) through glutaraldehyde linker as described previously. The same procedure was performed for conjugation of synthetic peptide and Bovine Serum Albumin (BSA) as a control. To examine the efficiency of conjugation, 10 µg TAZ-BSA were run on a 10% SDS-PAGE gel (Bio-Rad). The gel was stained with Coomassie Blue (Sigma). The change in mobility of conjugated BSA and appearance of the smear verified the efficiency of conjugation.
Immunization of mice: Three BALB/c mice were injected with TAZ-KLH, through 5 injections. The primary injection was administered intraperitoneal (I.P) with TAZ-KLH and Freund’s complete adjuvant. The second injection was done three weeks later, I.P with the antigen and incomplete Freund’s adjuvant. The last injections were administered every two weeks with the same conditions as the second injection. After the last injection, venous blood was obtained from an immunized mouse tail and the antibody titers were assessed by ELISA test.
Fusion and screening: Splenocytes from a hyperimmunized mouse were isolated and cell fusion was performed using mouse myeloma cells SP2/0 as fusion partner applying Kohler method 11. Hybridoma cells were obtained through the fusion of murine myeloid cells called SP2/0 with-BALB/c immunized mouse spleen cells. A mixture of cells (with the 1 ratio of SP2/0 to 5 ratio splenocytes) was washed with serum-free RPMI-1640 medium (Gibco, NY, USA), and then pre-warmed 50% polyethylene glycol (PEG 1500) (Sigma, Germany) was added with gentle shaking; after that the cells were washed again for the last time. For the next step cells were cultured in 96 well plates with Hypoxanthine Aminopterin-Thymidine (HAT, Sigma) as a differential medium for separating hybridoma cells. By limiting dilutions, the whole 96-well plate was screened by ELISA. To select productive clones, the limiting dilutions, were repeated four times.
Isotype determination and antibody purification: Iso-Strip mouse monoclonal antibody isotyping kit (Roche, Basel, Switzerland) was utilized for identification of anti-TAZ antibody, according to the manufacturer’s instruction. Anti-TAZ antibody was purified using antigen (peptide) affinity chromatography column.
Calculating the affinity constant of anti-TAZ mAb: The affinity constant (Kaff) of anti-TAZ mAb was measured through an ELISA method. The gradient dilutions were made (between 10 µg and 0.009 μg/ml) of TAZ peptide dissolved in Phosphate-Buffered Saline (PBS) in a 96-well plate. The ELISA plate was incubated at 37°C for 1 hr followed by overnight incubation at 4°C. The plate was washed three times in PBS/ Tween buffer for 5 min. for blocking, 150 μl of 2.5% BSA was added to each well and the plate was incubated at 37°C for 1.5 hr. Wells were then washed three times with PBS/Tween buffer for 5 min. TAZ-mAb sera were serially diluted in PBS and 100 μl were added to each well. The plate was incubated at 37°C for 1.5 hr and washed with PBS/Tween three times for 5 min. A rabbit anti-mouse Ig conjugated with horseradish peroxidase (Padzaco, Tehran, Iran) was used as the secondary antibody [100-μl (1:1000)], and the plate was incubated at 37°C for 1.5 hr and washed for three times. 100 μl of tetramethylbenzidine substrate (Sigma, St Louis, MO, USA) was added to each well and the plates were incubated at room temperature in a dark place. After 15 min, the reaction was stopped by adding 30 μl of stopping solution (0.16 M sulfuric acid). Optical density was measured at 450 nm using an ELISA reader. We further determined the binding capacity of TAZ mAb to TAZ peptide by calculating the affinity constant that reflected the antigen-antibody reaction using the following formula:
Affinity constant (KD) = dissociation constant (Kd) ÷ binding constant (Ka).
Specimen collection: Blood samples from 10 healthy donors were obtained with a signed informed consent letter and the study was approved by the Avicenna Research Institute local ethics committee.
Cell lines and culture conditions: Human epidermoid carcinoma cell line A-431, B-cell lymphoma cell line Raji, mammary gland adenocarcinoma cell line MCF-7, B lymphocytes RPMI 8226 and human foreskin fibroblast cell line HFFF-PI6 were grown and maintained in RPMI-1640 (Gibco, Grand Island, NY) containing 10% Fetal Bovine Serum (FBS, Gibco), 100 U/ml penicillin (ICN Biomedicals, Solon,OH), and 100 μg/ml streptomycin (Sigma, St. Louis, MO) at 37°C in a humidified incubator with 5% CO2 atmosphere.
RNA isolation and RT-PCR: For determining the positive and negative control cells, TAZ RNA expression in cell lines and Peripheral Blood Mononuclear Cells (PBMCs) were investigated. Primarily, total RNA of cell lines and PBMCs were extracted using RNA-zol (200 μl for 1×106 cell) (Sigma) solution according to the phenol-chloroform method. The DNase I (Sigma) was added to extracted RNA. The concentration of RNAs was assessed via a picodrop instrument (picodrop, UK). The cDNAs were synthesized through RT-PCR using 2 μg of RNA, 4 μl RT buffer (5X) (Fermentase), 2 μl of 20 pmol/μl dNTPs (Roche, Germany), 1 μl of 20 pmol/μl random hexamer primers (CinnaGen, Tehran, Iran), 1 μl Reverse Transcriptase enzyme (Fermentase), 0.5 μl RNase Inhibitor and 1.5 μl DEPC treated H2O followed by incubation in 42°C for 1 hr and later 10 min in 72°C. Before RT-PCR, RNAs were unfolded by incubation in Thermocycler for 5 min at 65°C and then cooled on ice.
The quality of produced cDNAs was checked by a PCR reaction with TATA Binding Protein (TBP) primers: 5´-TCTATTTTGGAAGAGCAACAAAG-3´as sense and 5´-ATGGTCTTTAGGTCAAGTTTACA-3´ as antisense. Then the qualified cDNAs were used in a PCR reaction to distinguish TAZ positive from TAZ negative samples. In this Polymerase Chain Reaction, TAZ-specific primers were utilized and performed in 35 cycles. The sequences of TAZ primers are 5´-ACGCAGGACCTAGACACAGAC-3´ as sense and 5´-GGTGTTGGTGATTCATCACGAGATT-3´ as antisense, respectively. PCR mixture was made of 7 μl master mix (Ampliqon), 1 μl of each sense and antisense primers (Sinaclon), 1 μl cDNA, and 5 μl DDW. For observing the DNA bonds PCR products were run on 1.5% agarose gel. VIII DNA ladder (Roche) loaded in one well for size detection of bands.
Validation of anti-TAZ mAb: Four different protein read-out systems were used for further characterization of anti-TAZ mAb. PBMCs were used as negative control and three cell lines (Raji, MCF-7, and A431) as positive samples.
Western blot: To evaluate the authenticity of anti-TAZ mAb in regard to the expected molecular weight of TAZ protein, Western blot was carried out in reducing conditions as described in detail previously 3. A polyclonal anti-TAZ antibody (Abcam, UK) was used as standard control. β-actin antibody was used as an internal control (Padzaco).
Flow cytometry: To evaluate the localization of TAZ protein, flow cytometry analysis was performed both at intracellular and cell surface staining using both produced anti-TAZ mAb and polyclonal anti-TAZ antibody (Abcam). Adherent cell lines A431 and MCF-7 were cultured and harvested by 0.1% EDTA. PBMCs and a B-cell line Raji were incubated with both antibodies for 1 hr and sheep anti-mouse and anti-rabbit conjugated to FITC was used as secondary antibody. An irrelevant mAb (Padzaco) was used as isotype control. The experiments were performed using a flow cytometer (Partec, Germany). The results were analyzed by FlowJo software.
Immunocytochemistry: Adherent cell lines were cultured on an 8 well microscope glass slides (Marienfeld, Germany), Raji cell line and PBMCs were suspended in PBS and placed on slides. Slides were air-dried and fixed by cold acetone. Then cells were blocked with 5% sheep serum for 30 min. Following incubation by anti-TAZ mAb (10 μg/ml) and irrelevant mAb (Padzaco) as isotype control. After 1 hr incubation, secondary antibody, sheep anti-mouse FITC-labeled antibody was added onto wells. Incubation time was 45 min in darkness. The cell nuclei were stained with 4',6-diamidino-2-phenyl- indole dihydrochloride (DAPI) (Calbiochem, USA) at 1 μg/ml concentration for 10 min. The cells were washed three times with PBS and then slides were mounted in 50% PBS-glycerol and analyzed via fluorescent microscope (Olympus, BX51, Japan).
Results :
Verification of TAZ-KLH conjugation: The efficacy of TAZ-KLH conjugation was examined via SDS-PAGE. The smear pattern of conjugated TAZ-KLH and the 67 kDa single band of BSA verified the efficiency of conjugation (Figure 1).
Characterization of anti-TAZ mAb: Using indirect ELISA, hybridoma clones were scre-ened based on their reactivity with the immunizing peptide. After excluding the non-reactive clones, ELISA screening revealed that the 1F3 clone showed higher reactivity when compared to other reactive hybridoma clones (Figure 2). Using the IsoStrip mouse isotyping kit, the isotype of produced anti-TAZ mAb was determined to be IgG2b with a kappa light chain.
Antibody purification and ELISA: Affinity column was used for purification of mAb 1F3 from hybridoma cell culture supernatant. Further, we calculated the affinity constant to evaluate the binding capacity of TAZ-mAb using the following formula: Affinity constant (kDa)=dissociation constant (Kd)/ binding constant (Ka). Results obtained from the Bia-core X100 (Learn more about SPR from ScienceDir-ect's AI-generated Topic Pages) analysis showed that the affinity constant of TAZ was 0.6×109 M-1.
RT-PCR analysis: First, the quality of the synthesized c-DNAs was checked. For this purpose, specific primers of TBP gene were used; the gene of which is part of the House-keeping genes and is commonly expressed in all cells. PCR products related to TBP gene expression in different cell lines are shown in figure 3A. It was observed that for all cDNAs synthesized in this test, the desired 400 bp band was created and subsequent tests could be performed on them. We performed quantitative real-time PCR reactions to detect TAZ mRNA expression in three different cell lines (A-431, MCF7, and Raji) and in PBMCs. The expression of TAZ was significantly elevated in all examined cell lines compared to the PBMC controls (Figure 3B).
Western blot analysis: For further characterization of anti-TAZ mAb (1F3), Western blot was performed on various cell lysates and PBMCs as a negative control. No reactivity was observed using both control anti-TAZ pAb and anti-TAZ mAb (1F3) on PBMC samples from healthy donors. Raji, A431, MCF7, MDA-MB231, and RPMI8226 were cell lines that the TAZ-mAb identified TAZ expression on them (Figure 4). B-actin antibody reactivity confirmed the amount of loading.
Flow cytometry: Cell surface staining of A431, MCF-7, Raji, MDA-MB-231 cell lines, demonstrated that 1F3 could react with an extracellular epitope of TAZ protein (Figure 5). Cell surface expression of TAZ was shown to be negative in HFFF-PI6 cells. No expression of TAZ was observed in PBMCs from healthy donors, confirming its specificity for TAZ in flow cytometry.
Immunocytochemistry: For further confirmation, cell surface staining of A431, MCF-7 and Raji cell lines was done (Figure 6). PBMCs were used as negative control cells. ICC confirmed that these cell lines express TAZ on their surfaces. The TAZ mAb indicated binding to cancerous cells at 10 μg/ml concentration.
Discussion :
Discovering novel and easy to target markers in cancer cells is of utmost importance to target and treat cancers. Ectopic expression of proteins (in terms of protein displacement in the cell) has been shown in many malignancies 12-15. These ectopically expressed molecules could be promising targets in cancer diagnosis and treatment due to the difference in their expression in normal and cancer cells. For the first time in this study, we demonstrated the ectopic expression of TAZ protein in cancer cell lines using our unique anti-TAZ antibody. Producing such specific monoclonal antibody against cell surface TAZ antigen provides an opportunity to specifically target cancer cells but not the normal cells which is the ultimate goal in cancer targeted therapy.
We utilized peptide-based antibody generation me-thod that gave us the opportunity to alter Post Translational Modifications (PTM). The antigen-antibody reactivity of anti-TAZ mAb was evaluated with ELISA test (Figure 2). Considering Western blot results the commercial anti-TAZ antibody could recognize TAZ protein in all five cell lines except for normal PBMC (as negative control) (Figure 4A) and our anti-TAZ mAb could detect TAZ in all of the cell lines except for HFFF-PI6 and normal PBMC. These results show that cytoplasmic and/or nuclear TAZ is expressed in HFFF-PI6 cells but our antibody is only capable of detecting TAZ on the cell surface. This is in accordance with the RT-PCR results depicted in figure 3 as no transcript was detected in normal PBMCs.
Flow cytometry results show that the commercial antibody could not detect the cell surface TAZ antigen in A431 cells but our anti-TAZ mAb could. Furthermore, the ICC images confirm these results for A431 cell line. This is in accordance with the Western blot analysis results mentioned earlier. Taking these results into account, we might conclude that our antibody specifically detects cell surface TAZ antigen in pathological conditions. This is due to the fact that our anti-TAZ mAb is against an epitope in TAZ protein different from the commercial antibody.
Amino acid sequence alignment of the immunogenic peptide of human, cow, mouse, hamster, pig, horse, dog and rat showed that the consensus sequence of PESFFKEPDSGSHSRQSSTDS could be recognized by anti-TAZ clone 1F3 (Table 1). Based on these results, TAZ protein from other species containing such epitope can potentially be recognized by this antibody. Furthermore, our antibody covers a part of the N-terminal part of TAZ involved in interaction with TEAD4 transcription factor leading to proliferation 16. Our antigenic peptide harbors a potential pathologic epitope. A mutation of a Serine to Alanine or Aspartic acid has been reported to alter such interaction 17, providing an opportunity to perform further functional assays utilizing this antibody.
Conclusion :
In conclusion, the produced antibody acts as a pan-species antibody with a broad range of applications like Western blot, immunocytochemistry, ELISA, and flow cytometry. Further investigation is necessary to identify the function of cell surface TAZ protein in vitro and in vivo.
Acknowledgement :
This work was supported by grants from Avicenna Research Institute, ACECR, Tehran.

Figure 1. Both KLH and BSA were simultaneously conjugated with TAZ peptide. TAZ-KLH conjugation was indirectly evaluated by SDS-PAGE gel with using TAZ+BSA instead. The observed smear indicates the quality of conjugation representing different molecular weight as a result of random covalent binding.
|
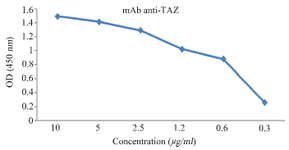
Figure 2. Titration of purified 1F3 in ELISA test. 1F3 was purified by peptide affinity column and its reactivity with the immunizing peptide was evaluated by ELISA.
|
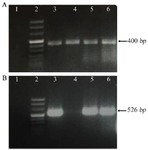
Figure 3. Gel electrophoresis results. A) The quality of the synthesized cDNA was evaluated by gel electrophoresis using TBP specific primers. Relative expression of TBP gene in the different cell lines by quantitative PCR. Positive samples have the desired 400 bp band. Negative sample (DDW) have no band. 1) No template control (NTC, DDW), 2) DNA size marker, 3) Raji, 4) Normal PBMC, 5) MCF-7, and 6) A431. B) Relative expression of TAZ mRNA in the different cell lines by quantitative PCR. Positive samples have the desired 526 bp band, PBMC or negative samples (DDW) have no band. Arrangement of loaded cDNAs: 1) No template control (NTC) (DDW), 2) DNA size marker, 3) Raji, 4) Normal PBMC, 5) MCF-1 6-A431.
|
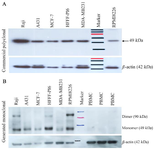
Figure 4. Investigation of TAZ expression in a number of cell lines via Western blot analysis. A) using commercial anti-TAZ pAb and Anti-β-actin mAb. Arrangement of loaded lysates: 1) Raji 2) A431 3) MCF-7 4) HFFF-PI6, 5) MDA-MB231 6) Size Marker 7) RPMI8226. B) Anti-TAZ mAb and Anti-β-actin mAb. All cells ex-pressed TAZ protein except PBMC. Arrangement of loaded lysates: 1) Raji 2) A431 3) MCF-7 4) HFFF-PI6, 5) MDA-MB231 6) RPMI8226 7) Size Marker 8, 9, 10) Normal PBMC.
|

Figure 5. Reactivity of 1F3 with cell surface TAZ on membranes of Burkitt's lymphoma, breast cancer and squamous cell carcinoma lines using flow cytometry technique. Values demonstrate the percentages of positive cells. 1F3 detected cell surface expression of TAZ in pathological conditions with no reactivity in normal conditions. Anti-TAZ mAb was applied on: A) A431, B) MCF-7, C) Raji D) HFFF-PI6 E) MDA-MB-231 G) Normal PBMC (anti-TAZ mAb) and anti-TAZ pAb was used on F) A431 (commercial pAb anti TAZ).
|
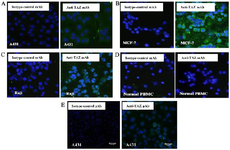
Figure 6. Immunocytochemistry (ICC) assay on A) A431 B) MCF-7 C) Raji cell lines, D) normal PBMCs and E) pAb anti TAZ on A431 cell line. Mouse monoclonal anti-TAZ antibody was used as a primary antibody and FITC-conjugated sheep anti-mouse antibody as a secondary antibody (Green). DAPI was used for counterstaining the nucleus (Blue).
|
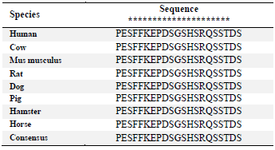
Table 1. The sequence alignment of immunogenic peptide from human TAZ to other species
The conserved amino acids are marked with asterisk (*).
|
|