Cloning and Expression of Leishmania infantum LPG3 Gene by the Lizard Leishmania Expression System
-
Pirdel, Leila
-
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
 Zavaran Hosseini, Ahmad
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, +98 21 82883090; zavarana@modares.ac.ir
Zavaran Hosseini, Ahmad
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, +98 21 82883090; zavarana@modares.ac.ir
-
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
Kazemi, Bahram
-
Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Rasouli, Manoochehr
-
Department of Immunology, Clinical Microbiology Research Centre, Shiraz University of Medical Sciences, Shiraz, Iran
-
Bandehpour, Mojgan
-
Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Soudi, Sara
-
Department of Immunology, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
Abstract: Background: Various prokaryotic and eukaryotic expression systems have been developed for the production of recombinant proteins. In the present study, we used a new protein expression system based on the Iranian Lizard Leishmania, a trypanosomatid protozoan as a host, for the expression of LPG3 gene from Leishmania infantum (L.infantum).
Methods: The LPG3 gene was cloned in the expression cassette for integration into the small subunit of the ribosomal RNA locus of Lizard Leishmania genome by electroporation. Expression of the recombinant LPG3 protein was confirmed by western blotting and immunofluorescence staining.
Results: Western blotting confirmed the expression and production of rLPG3 protein. Immunofluoresence analysis also revealed the staining throughout the cytoplasm of transfected parasites, indicating that the protein has been expressed.
Conclusion: These results demonstrate that Leishmania cells can be suggested an expression system for the production of recombinant LPG3 (rLPG3) to further research in vaccine designing against leishmaniasis.
Introduction :
The family Trypanosomatidae (Eugelen-ozoa, Kinetoplastida), which includes genera Leishmania, is one of the oldest groups of eukaryotes with a number of species that causes a range of debilitating or fatal diseases (1). About 200 million people are at risk of VL in 70 countries with estimated annual incidence of 500,000, and 50,000 deaths a year (2). Lack of an effective vaccine and the emergence of drug-resistant strains have made most intervention attempts extremely challenging for the identification of new targets for development of vaccine (3).
Therefore, the need for novel systems for the expression of recombinant proteins from protozoan parasites has become a pressing matter.
Leishmania produces a range of glycoconjugates containing phosphoglycan (PG) and two of the most studied abundant surface constituents are the GPI-anchored molecules lipophosphoglycan (LPG) and GP63 zinc metallo-protease (4) that play important roles in parasite survival and pathogenecity (5).
LPG3 is one of the class II LPG genes that encodes the Leishmania homolog of the mammalian Endoplasmic Reticulum (ER) chaperone GRP94, which belongs to HSP90 family (6). It is involved in variety of processes including antigen presentation, folding and assembling of proteins, and secretory pathway. It also contains antigenic and immunogenic properties similar to other conserved antigens of the parasite (7,8).
Although a variety of prokaryotic and eukaryotic expression systems have been developed for the synthesis of recombinant proteins such as bacteria, yeast, fungi, insect cells, mammalian cells, transgenic animals, and transgenic plants, none is universally applicable (9). Leishmania has many advantages to use in biotechnological applications. Recently, Leishmania tarentolae, a nonpathogenic parasite of the gecko Tarentolae annularis, has been established as a new eukaryotic expression system for the production of recombinant proteins. In addition, it has already been used successfully for the expression of erythropoietin (10), tissue plasminogen activator (11), IFN- (12), human laminin-332 (13) and proprotein convertase 4 (a member of Ca2+ dependent mammalian subtilases) (14). Most recently, the Iranian Lizard Leishmania has been used as an expression system for producing rFVII (15).
Here, cloning and expression of LPG3 gene from L.infantum in the Iranian Lizard Leish-mania are carried out for producing rLPG3 as a preliminary step for further investigation into vaccine development.
Materials and Methods :
Amplification of LPG3 gene: Genomic DNA of L.infantum, cultivated in RPMI 1640 medium (Gibco-BRL, UK) and supplemented with 10% heat-inactivated FBS (Gibco-BRL, UK), 10 mM HEPES, 15 g/ml hemin, 100 U/ml penicillin, and 100 g/ml streptomycin (Gibco-BRL, UK) at 26C, was extracted by phenol: chloroform: isoamyl alcohol extraction (1:1:1) and ethanol precipitation.
Then the LPG3 gene was amplified with forward primer, F-LPG3 (5 AGATCTATGG CGAACTCGAGCTTGC3) and reverse primer, R-LPG3 (5GCTAGCCAGATCGTCCT CGCCGACTG3), containing BglII and NheI restriction sites in each 5' end (underlined). PCR reaction was performed under the following conditions: 95 oC (5 min), 35 cycles of 95oC (1 min), 58oC (1 min), 72oC (2 min) and 72oC (10 min) for final extension. The 2316 bp desired band was cleaned up by QIAquick Gel Extraction Kit (Qiagen, USA) following the manufacturer's protocols.
Cloning of LPG3 gene:The purified PCR product was cloned in pTZ57R cloning vector using InsT/AcloneTM PCR Product Cloning Kit (Fermentas, Lithuania) following the manufacturer’s procedures and transformed into competent Escherichia coli (E.coli) DH5. The transformed cells were screened for the presence of recombinant plasmid with the LPG3 insert by gene-specific PCR and analysis with BglII and NheI restriction enzymes. Isolated positive clones were sequenced using M13 forward and reverse primers by MWG Operon's Sequencing Service (Germany). The insert was removed by BglII and NheI digestion and subcloned into the BglII-NheI insertion site of Leishmania expression vector pLEXSY-hyg2 to create the recombinant pLEXSY-LPG3 plasmid. The presence of the LPG3 gene in pLEXSY-hyg2 was confirmed by BglII and NheI restriction enzymes and PCR amplification.
Cultivation and transfection of Lizard Leishmania: Lizard Leishmania promastiogtes were cultivated in RPMI 1640 supplemented with 5 g/ml hemin, 100 IU/ml penicillin and 100 g/ml streptomycin (sigma, USA) at 26oC (16). For integration of the expression cassette into the 18S ribosomal RNA (ssu) locus, the pLEXSY-hyg2 plasmid containing LPG3 gene was digested by SwaI restriction enzyme (Fermentas, Lithuania) and 10 g of the heavier fragment was transformed into cultivated Lizard Leishmania promastigotes by electroporation in vitro. Transgenic cells were selected as single colonies on the supplemented RPMI 1640-agar medium containing 100 g/ml hygromycine B (Jena Bioscience, Germany) as a selective antibiotic. To confirm the integration of the LPG3 containing cassette into the Leishmania genome, PCR was performed on the genomic DNA of wild type and transgenic cells with LPG3 forward and ssu reverse primers (Jena bioscience, Germany):
LPG3 Forward: 5`- AGATCTATGGCGAAC TCGAGCTTGC3 -3'
ssu Reverse: 5-CTGCAGGTTCACCTACAG CTAC -3
Western blot analysis: The wild type and transgenic cells were harvested and suspended in 50 l of SDS-PAGE sample buffer containing 1 Mm PMSF. Then they were sonicated twice with 70 Hz for 20 s and boiled for 5 min. Twenty l of the obtained sample was loaded on 10% polyacrylamide gel. Western blotting was performed on similarly prepared acrylamide gel and electrophoretically transferred onto the nitrocellulose membrane. After UV cross-linking for protein fixation, the membrane was blocked with 5% skim milk at room temperature. Mouse monoclonal HRP-conjugated anti-His tag antibody (Abcam, UK) was used in 1:1000 dilutions for 2 hr at room temperature. The protein band was detected by Diamino Benzoic Acid (DAB) and H2O2.
Indirect immunofluorescence assay (IFA): Lizard Leishmania promastigotes were fixed with 4% paraformaldehyde for 15 min at room temperature, washed three times in PBS, permeabilized by 0.1% Triton X-100 for 5 min at room temperature and washed three times in PBS. Subsequently, the cells were blocked for 30 min in PBS containing 10% goat serum. After three washes in PBS, the cells were incubated overnight with mouse monoclonal anti-His tag antibody diluted in PBS/10% goat serum (1:40), and FITC conjugated anti mouse IgG antibody diluted in PBS/10%goat serum (1:100) for 1 hr at room temperature. After three washes in PBS and counterstaining with DAPI (4, 6-diamidino-2-phenylindole), the cells were mounted in glycerol/PBS solution (1:1). Finally, they were examined for fluorescence under Nikon immunofluorescence microscope.
Results :
Subcloning of LPG3 gene: A PCR reaction with LPG3-specific primers on the genomic DNA of L.infantum resulted in a single band with the expected size of 2316 bp. The desired band was ligated into T/A cloning vector, pTZ57R, excised by BglII and NheI enzymes (Figure 1) and subcloned into the expression vector, pLEXSY-hyg2 containing antibiotic resistance gene, hygromycin B (Figure 2). The sequence of the LPG3 was confirmed by restriction analysis and DNA sequencing (GenBank: HQ400675. 1). BLAST assessment of the obtained sequence showed 98% identity with the data available in the GenBank (Figure 3).
Transfection of lizard leishmania cells: The hyg2-LPG3 fragment containing one copy of the LPG3 obtained from the digestion of pLEXSY-LPG3 with the SwaI restriction enzyme was used for integration into the small subunit of Lizard Leishmania rRNA gene for homologous recombination by electroporation. After transfection, hyg-resistant cells were selected on semisolid RPMI 1640-agar medium containing 100 g/ml hygromycin and cultured in supplemented RPMI 1640 containing 100 g/ml hygromycin B for the second selection. Integration of the expression cassette into the ssu locus was confirmed by PCR on the genomic DNA of the recombinant cells using forward LPG3 and reverse ssu primers, which generated a 3.5 kb band, demonstrating the occurrence of homologues recombination in the 18 s rRNA locus with the right orientation (Figure 4).
Western blotting: Expression of rLPG3 in Lizard Leishmania was investigated by western blot analysis. The expressed rLPG3 was detected in the lysate of transfected cells with mouse monoclonal HRP-conjugated anti-His tag antibody as a single band with an apparent MW of 97 kDa, which is slightly high due to the addition of a hexa-His tag sequence whereas the lysate of wild type cells did not react with monoclonal antibody (Figure 5).
Indirect immunofluorscence: It was necessary to determine the percentage of the transfected and selected cells that expressed the rLPG3 protein. As shown in figure 6, immunofluorescence analysis on the fixed and permeabilised Lizard Leishmania promastigotes showed that rLPG3 is present in all of the cells at approximately equal levels. This also demonstrates that LPG3 is largely present throughout the cytoplasm of the cells, indicating the cells expressing the rLPG3 protein.
Discussion :
In the current study, we have used a non-pathogenic trypanosomatidae, Lizard Leishmania, to represent a beneficial eukaryotic expression system for the production of recombinant proteins. One reason for using trypanosomatidae species is that high level expression of protein-coding genes driven by RNA polymerase I is obtained by integration into the 18 s rRNA locus (17). The main advantages of Leishmania expression system are as follows: (i) inexpensive growth condition, (ii) fast growth rate, (iii) safety for human, (iv) production of recombinant proteins and (v) posttranslational modification of target proteins with a mammalian-type N-glycosylation pattern and correctly protein folding (18) result to make Lizard Leishmania an attractive host for high level production of heterologous proteins such as human p53 (9), erythropoietin (10), IFN- (12), and human tissue plasminogen activator (19). Thus, the expression cassette containing the LPG3 gene was integrated into the ssu rRNA locus, which could enhance the expression level. Among the posttranslational modifications, glycosylation can be critical, not only for immunogenicity that it can enhance or conceal immunogenic epitopes but also to modulate biological activity (20). Since Trypanosomatidae species naturally produce large amounts of glycoproteins, then this feature could be advantageous for the production of glycosylated proteins (21). For these reasons, Lizard Leishmania has attracted extensive attention to produce this valuable molecule (LPG3) in a different expression system.
The results showed that rLPG3 can be expressed in Lizard Leishmania to a level that allows detection by western blotting in the transfected cells, but this protein was absent in the wild type cells. The critical role of LPG3, homolog of the mammalian ER chaperon GRP94, is restricted to the synthesis of glycoconjugates implicated in parasite virulence, including LPG (lipophosphoglycan) (6). In addition, due to high immunogenicity of LPG3, it can be used as a valuable molecule for diagnostic purposes and a promising candidate for vaccine development against leishmaniasis (7,8).
Alignment of the amino acid sequence of LPG3 with the native signal sequence revealed several conserved regions, including the C-terminal ER retention signal, to localize in the ER. Moreover, immunofluorescence staining determined that the expressed protein was largely present throughout the cytoplasm of parasites, indicating the cells ability to express the recombinant protein. The Iranian Lizard Leishmania expression system has also been used to produce human coagulation factor VII with biological properties (15). This confirms that Lizard Leishmania represents potential advantages including full glycosylation, disulfide bond formation and proper folding.
Conclusion :
In conclusion, this study has demonstrated that Leishmania cells can be used as a suitable host for the production of rLPG3 protein. We could successfully express the rLPG3 in this host to perform the future experiments on the usage of rLPG3 as a vaccine candidate by different vaccination strategies (Protein/ Protein and DNA/Protein) against leishmaniasis.
Acknowledgement :
The authors would like to express their appreciation to the kind help of Dr Mehdi Mohebali (School of Public Health, Institute of Public Health Research, Tehran University of Medical Sciences) for supplying L. infantum, and also the staff of Clinical Microbiology Research Centre of Shiraz University as well as Cellular and Molecular Biology Research Center, Shahid Beheshti University of Medical Sciences for useful guidelines. The authors have declared that no competing interests exist.
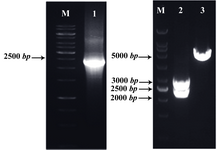
Figure 1. Detection of the recombinant plasmid pTZ57R vector-LPG3 by PCR and restriction enzyme digestion: amplified LPG3 gene (Lane 1), restriction analysis of pTZ57R-LPG3 vector (Lane 2), undigested vector (Lane 3), and 1 kb DNA size marker (Lane M). The products were electrophorased on 1% agarose gel
|
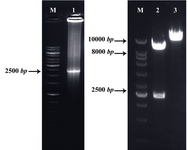
Figure 2. Detection of the recombinant pLEXSY-LPG3 vector by PCR and restriction enzyme digestion: amplified LPG3 gene (Lane 1), restriction analysis of pLEXSY-LPG3 vector (Lane 2), undigested vector (Lane 3), and 1 kb DNA size marker (Lane M). The products were electrophorased on 1% agarose gel
|

Figure 3. Sequence alignment of the deduced amino acid sequence of L.infantum LPG3 with other related proteins. The sequences were aligned with CLC protein workbench 5.5.1 software. The identical residues are indicated by dots. The boxes indicate the signal sequence at the N-terminus (box I), ATPase_c domain (box II), dimerization domain (box III), and the C-terminal ER retention signal (box IV). The GXXGXG motif (grey letters in box II) is also conserved in all Hsp90 family proteins. Potential N-linked glycosylation sites are indicated by gray underlines and protein kinase C phosphorylatin sites are marked by asterisks. Hyphens represent the introduced gaps for the optimum alignment.
|
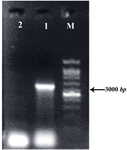
Figure 4. Confirmation of genomic integration into the ssu locus of Lizard Leishmania by diagnostic PCR with the forward LPG3 and ssu reverse primers: transfected cells with pLEXSY-LPG3 vector (Lane 1), wild type cells (Lane 2), and 1 kb DNA size marker (Lane M). The products were electrophorased on 1% agarose gel
|
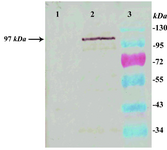
Figure 5. Western blot analysis of the transfected Leish-mania cells lysate: lysate of wild type cells (Lane 1), lysate of cells transfected with pLEXSY-LPG3 expression vector blotted with anti-His tag monoclonal antibody (Lane 2), and molecular weight markers (Lane 3)
|
Figure 6. Indirect imunofluorescence analysis: (A, B, C), wild type cells; (D, E, F), transfected cells; (A, D), phase-contrast images; (B, E), DAPI staining, indicating the localization of DNA in the nucleus and kinetoplast in blue, and (C, F) FITC staining (green) using the anti-His tag monoclonal antibody diluted (1:40) for wild type and transfected cells ( 40).
|
|