The Anticonvulsant and Neuroprotective Effects of Walnuts on the Neurons of Rat Brain Cortex
-
 Asadi-Shekaari, Majid
Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran, Tel: + 98 341 2264152; Email:majidasadi@kmu.ac.ir
Asadi-Shekaari, Majid
Neuroscience Research Center, Kerman University of Medical Sciences, Kerman, Iran, Tel: + 98 341 2264152; Email:majidasadi@kmu.ac.ir
-
Neuroscience Research Center, Kerman University of Medical Sciences,, Kerman, Iran
-
Arab Nejad, Fatemeh
-
Member of Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran
-
Namazian, Elaheh
-
Member of Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran
-
Eslami, Azam
-
Department of Physiology, Science and Research Branch, Islamic Azad University, Shiraz, Iran
Abstract: Background: Epilepsy is a chief communal health problem. Antiepileptic drugs only provide symptomatic treatment. Walnut Kernels (WK) have high concentrations of phenolic compounds, which have beneficial effects on human health because of their antioxidant and anti-atherogenic properties. The present study was designed to evaluate the efficacy of WK supplementation for the prevention of experimental epilepsy in male rats.
Methods: Wistar adult male rats were divided into three groups: a control group (PTZ injection, fed with ordinary food), experimental group (PTZ injection, fed with WK) and a sham group (no PTZ injection, only for histological studies). Pentylenetetrazole (PTZ) was administered after the prescribed time.
Results: WKs displayed anti-epileptogenic properties, and WK supplementation was associated with increased seizure threshold and reduced mortality in the experimental group versus controls.
Conclusion: Use of WK may be helpful in prevention of PTZ-induced seizure and its further neurodegeneration in male rats.
Introduction :
Epilepsy is a major communal health problem, affecting approximately 4% of individuals over their lifetime (1). Antiepileptic drugs only provide symptomatic treatment, devoid of having any influence on the course of the illness. There is a great need for the development of alternative therapeutic approaches that prevent epilepsy (2).
Several herbal products with direct antioxidant activity have been shown to possess anticonvulsant activity (3,4). WKs have high concentrations of phenolic compounds, which have beneficial effects on human health because of their antioxidant, anti-atherogenic and neuroprotective properties (5,6).
The current study was designed to evaluate the efficacy of WK supplementation for the prevention of experimental epilepsy in male rats.
Materials and Methods :
Plant material
The WKs were collected from Rabor area, Kerman Province, Iran in September, 2010. A voucher specimen was deposited at the herbarium of Kerman faculty of Pharmacy, Kerman University of Medical Sciences (No. 1401-1).
Animals
Wistar rats were divided into three groups: control (fed with ordinary food 20 g daily for two months and PTZ administered after prescribed time) (n=17), experimental (fed with WK, 6% of food intake for two months and PTZ administered after prescribed time) (n=8) and sham (intact) (no PTZ injection, only for histopathological study) (n=4). Animal experiments were performed in accordance with Kerman Neuroscience Research
Center (KNRC) ethics committee guidelines.
Anticonvulsant study
Each animal was restrained in a clear Plexiglas cylinder and a 27-gauge infusion needle was put in the tail vein, which was held tightly in place to prevent it from moving for the duration of convulsive activity. The animal was observed during infusion and the first convulsion sign was recorded as the seizure threshold. The end point of infusion was the manifestation of tonic-clonic spasm. The sum of PTZ (2 mg/kg) required at threshold or tonic-clonic convulsion was calculated by following parameters: duration of convulsant infusion, infusion speed, the weight of the animal and the concentration of convulsant in the injected liquid (7).
Assessment of cortex neuronal injury
Light microscopy: Under deep anaesthesia with cholral hydrate (400 mg/kg), 48 hours after administration of PTZ, the animals were perfused. The brains were processed for light microscopy studies according to routine procedures. Neuronal injury in the cortex was measured by staining sections with haematoxylin and eosin using the standard method (8).
Electron microscopy: The animals were transcardially perfused through the left ventricle and their brains were processed for ultrastructural study according to the routine procedure. Ultrathin sections (80 nm) were studied using an electron microscope (Philips EM300).
Statistical analysis: Differences in measured parameters among different groups were analyzed using the one-way ANOVA and Student’s t-test. Statistical differences were defined as p<0.05.
Results :
Effects of WK on PTZ induced seizures
Pre-treatment with WKs significantly increased the seizure threshold induced by PTZ (Figure 1).
Mortality
Pre-treatment with WKs decreased the mortality rate after PTZ administration (12.5 vs. 76.47%).
Effects of WK on neuronal injury induced by PTZ injection
Light microscopy: WK pre-treatment reduced neuronal injury induced by PTZ (21.49 vs. 76.31%) (p<0.01) (Figure 2).
Electron microscopy study: In the intact group, the morphology of the neurons in the cortex was normal (Figure 3A). In contrast, most of the cortical neurons in the control group showed severe degenerative changes, including: cytoplasm darkening, mitochondrial swelling, and shrinkage of nuclei (Figure 3B). Electron microscopy revealed that neuronal architecture was nearly intact in the WK pre-treated group (Figure 3C).
Discussion :
In the present study, WK pre-treatment was associated with significant anticonvulsant and neuroprotective activities in PTZ-induced seizures. WK contain crucial fatty acids including Alpha-Linolenic Acid (ALA), Linoleic Acid (LA), and oleic acid (9).
Reactive Oxygen Species (ROS) have been concerned in the development of seizures under pathological conditions, and have been linked to neurodegeneration induced by seizure (10). In addition, there are a rising number of different studies regarding the role of Nitric Oxide (NO) in the pathophysiology of disorders such as stroke, trauma and seizure disorders (11,12). In epilepsy, NO is also considered as an important pathogenic factor; and NO is reported to play a function in the mechanisms underlying seizure induction and progression (13). Consistent with this, dramatic (five-fold) elevations in NO production were found during seizures induced by penthylenetetrazole (14).
A recent study demonstrated that walnut extracts reduced production of NO, TNF-α and the expression of inducible NO synthase, in BV-2 microglial cells activated by lipopolysaccharide (LPS) (15). Another study has shown that fatty acids decrease NO production in macrophages stimulated by LPS through iNOS protein expression (16).
According to the obtained data, WK can protect neurons against oxidative stress induced by seizure. To date, the exact anticonvulsive and protective effects of WKs remain unknown. However, support for these effects comes from the fact that the fatty acid components of WKs can inhibit NO production in seizure conditions. In addition, some studies showed that polyunsaturated fatty acids can modulate neuronal excitability and lower the susceptibility to epileptic seizures (17,18). Walnuts have many unsaturated fatty acids that may act through this mechanism. Furthermore, the neuroprotective effects of WKs may be related to their known neuroprotective components, such as melatonin, polyphenols, vitamin E, and folate. Further studies, focusing on NO production and iNOS expression after administration of PTZ, accompanied by a WK diet, are needed to clarify the precise mechanisms involved.
Conclusion :
Use of WK may be helpful in prevention of PTZ-induced seizure and its further neurodegeneration in male rats.
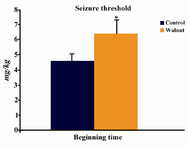
Figure 1. Effect of WK pre-treatment on PTZ-induced sei-zures. The mean±S.E. is presented for seizure threshold values. *p<0.05
|
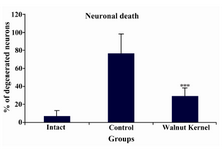
Figure 2. Neuroprotective effects of WK pre-treatment on PTZ-induced seizures in male rats
|
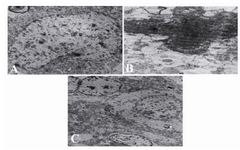
Figure 3. Electron micrograph of cortical neurons from in-tact, control, and WK pre-treated groups. Normal ultrastruc-ture is visible in intact neurons. Seizure results in severe de-generative changes in cortical neurons (part B). Cortical neurons from the WK pre-treated group show some degener-ative changes, but the whole ultrastructure was maintained (part C). Magnification in (A) and (B) is 8900 × and in (C) is 3900
|
|