Construction and Stable Expression of a Truncated Human Receptor Tyrosine Kinase Ror1 (Ror1-ECD)
-
Forouzesh, Flora
-
Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Shakeri Tabarian, Samira
-
Department of Antigen and Antibody Engineering, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Emami, Shaghayegh
-
Department of Antigen and Antibody Engineering, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Jeddi-Tehrani, Mahmood
-
Department of Antigen and Antibody Engineering, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Hadavi, Reza
Reza Hadavi, M.Sc., Depart-ment of Antigen and Anti-body Engineering, Mono-clonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020 Fax: +98 21 22432021 E-mail: rezajahrom@gmail.com
Hadavi, Reza
Reza Hadavi, M.Sc., Depart-ment of Antigen and Anti-body Engineering, Mono-clonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: +98 21 22432020 Fax: +98 21 22432021 E-mail: rezajahrom@gmail.com
-
Department of Antigen and Antibody Engineering, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Rabbani, Hodjattallah
-
Department of Antigen and Antibody Engineering, Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Expression of receptor tyrosine kinase Ror1 in a wide variety of cancers has emerged as a new era focusing on targeting this receptor in cancer therapy. Our preliminary re-sults indicate the presence of a truncated transcript of Ror1 in tumor cells. The trun-cated Ror1 encompasses extracellular and transmembrane domains, lacking catalytic kinase domain (Ror1-ECD). As enzyme activity is highly dependent on the catalytic domain, we were wondering how this transcript and its encoded protein could play a possible role in tumorigenesis. To understand the function of this truncated transcript and whether or not the encoded protein translocates to the cell surface, we construct-ed a mammalian expression vector containing exon 1 to exon 8 of human Ror1 gene as a model system. The encoded protein by this construct covers the entire extracellular and transmembrane domains of Ror1. The Chinese Hamster Ovary Cell line (CHO) was used for transfection. Our results showed that this construct could express Ror1-ECD at protein level and also the protein could effectively translocate to the surface of transfected cells. Such model may suggest that a proportion of Ror1 molecules ex-pressed by tumor cells are not full-length Ror1. This notion may be considered when applying flow cytometry using antibodies against Ror1 for screening of tumor cells in order to avoid any miscalculation in the number of Ror1 molecules expressed by tumor cells. Furthermore, such expression may bring about assumptions on functional roles of Ror1-ECD in tumorigenesis, which requires extensive functional studies.
Introduction :
Receptor tyrosine kinases are critical signal-transduction mediators regulating essential cel-lular activities including growth, differentiation and migration, as well as survival and death (1). Tyrosine kinase-like orphan receptors (Ror) are predominantly located in the plasma membrane (2). The extracellular part of Ror proteins contains an immunoglobulin domain, a cysteine rich domain, also called a frizzled domain and a kringle domain (3-7). Ror receptors possess a tyrosine kinase do-main in their intracellular parts (8,9). The human Ror1 gene is located on chromosome 1p31-p32 encoding a protein of 105 kDa (10-12).
Others and we have recently reported expres-sion of Ror1 in a variety of malignancies in-cluding acute lymphoblastic leukemia, Chronic Lymphocytic Leukemia (CLL), mantle cell lym-phoma, marginal zone lymphoma, diffuse large B-cell lymphoma, follicular lymphoma and also renal cancer (13-20). The widespread expression of Ror1 in different malignancies with no expression in normal adult tissues makes it a suitable can-didate for targeting the cancer cells. In an attempt to identify possible variants of Ror1, we isolated a transcript variant of Ror1 from blood of a CLL patient encompassing the extracellular and trans-membrane domains lacking the kinase domain. Such variant has been reported at transcript level (GenBank locus NM-001083592) and protein level of 50 kDa band in patients with CLL (11).
To understand the functional role of this isomer, we designed a construct containing exons 1-8 of Ror1 and transfected this construct into Chinese Hamster Ovary (CHO, CCL-61, ATCC) cell line. Here we describe establishment of a cell line stably expressing the extracellular part of human Ror1 (Ror1-ECD) localized to cell membrane.
Materials and Methods :
Vector construction
Ror1-ECD was PCR amplified using a human full-length cDNA clone EN1031_D08 Ror1 gene (Origene Technologies, MD) as template and primers with appropriate restriction sites. A sense primer was GGTACCGCCACCATGCACCGGC CGCGCCGCCGC with KpnI restriction site plus KOZAK sequences (GCCACC) and an antisense primer was TCTAGACTACTTGGGTTTATATG ATTCAGC with XbaI restriction site plus TAG as a stop codon.
PCR was carried out in a 25 μl reaction [1 μl of template, 1 μl of forward and reverse primers
(10 pmol/μl), 1 μl dNTPs (10 mM), 4 mM MgCl2, 2.5 μl 10×buffer, and 1 Unit Taq DNA polymer-ase (Invitrogen, USA)]. The mixture was heated to 95°C for 5 min and then amplified for 35 cycles: 94°C for 30 s, 64°C for 30 s and 72°C for 1 min. The PCR products were purified from 1.5% agarose gel and cloned into pGEM-T Easy Vector (Promega, USA). Several clones were selected and plasmids were prepared. Plasmids were di-gested with KpnI and XbaI restriction enzymes. The fragment was sub-cloned into pCMV6-Neo vector (Origene Technologies). Authenticity of the construct was verified by DNA sequencing. Recombinant plasmids were transformed and pro-pagated in Escherichia coli (E.coli) JM109 (Pro-mega). Plasmid Maxiprep was performed. For transfection the construct was linearized using XmnI restriction enzyme.
Stable transfection of CHO cells
CHO cells were cultured in RPMI-1640 supple-mented with 10% fetal bovine serum, strepto-mycin (100 μg/ml) and penicillin (100 U/ml) (In-vitrogen) at 37°C in a humid atmosphere with 5% CO2.
Then 106 CHO cells were seeded per each 6-well plate. After 24 hours 10 μg of linearized plas-mid containing the Ror1-ECD as well as pCMV6-Neo empty vector were transfected into CHO cells (with 50-70% confluency) using Polyplus trans-fection-jetPEI (Bioparc, France) according to the manufacturer's instructions. In transient transfec-tion, proteins were analyzed at 48 hr after DNA introduction. To establish stable lines, CHO-trans-fected cells were treated with G418 (850 μg/ml, Invitrogen). Stable transfectants were generated after 3 to 4 weeks post-transfection and cell cloning was performed using limiting dilution method. Expanded clones were checked for ex-pression of Ror1-ECD by Western blot and flow cytometry.
Western blot
Transfected cells were prepared in lysis buffer [1% Nonidet P-40, 0.1% SDS, 1% sodium deoxy-cholate, 25 mM Tris-HCl, pH=7.2, 150 mM NaCl and 100 μl/ml protease inhibitor cocktail (Sigma, MO)]. After 20 min, Insoluble material was re-moved by centrifugation (13000 rpm, 15 min) at 4°C. Concentration of protein was measured by BCA Protein Assay Kit (Thermo Fisher Scientific, Germany) following manufacturer's instructions. Twenty micrograms of protein was used for im-munoblotting. Sample buffer (0.5 M Tris-HCl pH=6.8, 10% SDS, 0.5% bromophenol blue and 50% glycerol) was added to the lysate (1:4). Sam-ples were boiled at 100 °C for 5 min, then loaded on a 10% SDS-PAGE (21). Proteins were trans-ferred to polyvinylidene fluoride (PVDF) mem-branes (Millipore Corp, MA) (22) and were blocked with PBS-0.1% Tween 20 buffer (PBS-T) plus 5% nonfat milk powder (blocking buffer) at room temperature for 1 hr.
The membranes were incubated for 1 hr at room temperature with a 0.2 μg/ml goat anti human Ror1 antibody (R&D Systems, MN). After four times washing, the membranes were in-cubated with 1:2000 dilution of rabbit anti-goat- HRP conjugate (Dako Cytomation, Denmark). After thorough washing, bands were visualized by ECL reagent (GE Healthcare, Sweden) according to the manufacturer's instructions and the mem-branes were exposed to X-ray film.
Cell surface flow cytometry
CHO-transfected and untransfected cells (1x106 cells/ml) were resuspended in FACS buffer (PBS, pH=7.2 and 0.1% sodium azide). Two μg/ml goat anti-human Ror1 antibody was added and the tubes were incubated on ice for 1 hr. Cells were washed three times in FACS buffer and incubated with a 1:100 dilution of rabbit anti-goat-FITC con-jugate for 30 min at dark. After washing, fixation buffer (1% formaldehyde in PBS and 0.1% so-dium azide) was added. The analyses were per-formed based on two negative controls. The mean value of fluorescence intensity of 10000 cells was determined by FACS, PAS system version 2.4e (Dako Cytomation). FlowMax software (Dako Cyto-mation) was used for analysis of data.
Results :
Ror1-ECD was successfully cloned into pGEM-T easy vector by PCR (Figure 1A). Seven white col-onies were selected for PCR screening of which all were positive (Figure 1B). Three selected posi-tive colonies were subjected to double digestion using KpnI and XbaI restriction endonuclease. A representative experiment is shown in figure 1C. The excised Ror1-ECD fragment was ligated into pCMV6-Neo. The sequencing of two clones ver-ified cloning of entire exons 1-8.
Western blot and flow cytometry techniques were used to verify the expression of Ror1-ECD construct. Expression of Ror1-ECD protein in tran-siently transfected cells was analyzed with anti-human Ror1 antibody. Two bands of ~55 and
60 kDa proteins were detected in CHO transfected cell lysate with no expression in control samples confirming the integrity of the construct. The
~55 kDa band was much weaker than the 60 kDa band in transiently transfected cells (Figure 2A) in contrast to strong band in stably transfected CHO cells (Figure 2 B). Two final stable clones obtain-ed expressing Ror1-ECD with similar pattern to transient cells (Figure 2C).
Cell surface expression of Ror1-ECD was determined by flow cytometry showing more than 98% positivity (Figure 3).
Discussion :
Baskar et al (11) demonstrated expression of a truncated Ror1-ECD as one single band of ~55kDa in CLL tumor cells. Based on the molecular weight of this fragment as well as interaction of antibody (against extracellular domain of Ror1) we as-sumed that this fragment might only encompass the extracellular part of Ror1. To verify this hypo-thesis we expressed Ror1-ECD in CHO cells. The results showed a single weak band of 60 kDa in our transiently transfected cells (Figure 2A). The final stable lines showed two bands of 60 and
~55 kDa bands similar to what was reported by Baskar et al (Figures 2B and 2C). The 5 kDa dif-ference in molecular weight might be due to post-translational modifications (mainly glycosylation) leading to a higher molecular weight, which is more potent to glycosylation in CHO cells (with normal origin) than tumor cells. Moreover, ex-pression of such construct at protein level verifies the suitability of the selected strategy including adding the KOZAK consensus sequences at 5´site of start codon.
The successful surface expression of extracel-lular part of Ror1 lacking the kinase domain in-dicates that such structure may also be expressed on cell surface of leukemic cells from CLL pa-tients as well as cells from other hematological malignancies. The translocation of expressed pro-tein to the cell surface may imply that the 55 kDa fragment is also expressed in the surface of CLL cells (11). Based on such data we may assume that some of the Ror1 molecules expressed on the sur-face of tumor cells might actually represent a trun-cated Ror1-ECD lacking kinase domain. This may bring up the question that what is the function of such truncated Ror1 in tumor cells. To address this question, further functional analyses are war-ranted.
Conclusion :
Ror1 is a receptor tyrosine kinase with an intra-cellular catalytic domain. All enzymatic activity of Ror1 depends on the kinase domain. Apparent-ly, expression of the truncated Ror1 on the surface of tumor cells does not hamper the function of full-length Ror1 in its intracellular signaling as the cells are surviving. We assume that truncated Ror1 may have a synergist role in cell survival, increasing the importance of such variant in tumor development. Moreover, such structure may also play a role in non-pathologic conditions by in-hibiting the signaling via adsorbing the Ror1 li-gands, a notion to be considered. As such trun-cated structure includes the ligand binding do-mains may assist in binding to the ligands already exist in the extracellular matrix at in vivo con-ditions, assisting signaling in a ligand-dependent manner in association with the full-length Ror1. Furthermore, we may use this stable line as an im-munogenic source for production of monoclonal antibodies against limited part of Ror1 for thera-peutic purposes (23).
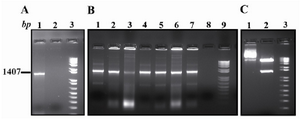
Figure 1. PCR amplification and cloning. A) Ror1-ECD PCR amplification (Lane 1). B) Colony PCR screening for identifying the recombinant clones (Lanes 1-7). C) Lane 1 is undigested and Lane 2 is KpnI/XbaI double di-gestion of a representative recombinant clone. The mar-ker is a1 kb plus DNA ladder
|
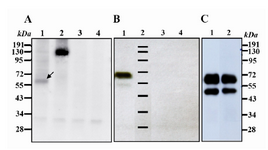
Figure 2. Western blot analysis using goat anti human Ror1 at non-reducing conditions. A) Lane 1: CHO cell line transiently transfected with Ror1-ECD construct. The arrow shows a weak band of around 60 kDa. Lane 2: CHO cells transfected with full-length Ror1 construct as a positive control. Lane 3: CHO-transfected with pCMV6-Neo empty vector. Lane 4: Untransfected CHO cells B) Lane 1: cell lysate from a stable CHO transfected with Ror1-ECD. A weak band ~ 55kDa appears which may represent the unglycosylated variant. Lane 2: Protein marker. Lane 3: CHO transfected with pCMV6-Neo empty vector. Lane 4: Untransfected CHO cells.
C) Two final clones obtained after cell cloning of stable transfectants. Lane 3: protein marker
|
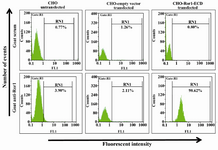
Figure 3. Cell surface staining of stably transfected CHO cells. Upper panel: Goat serum used as primary antibody. Lower panel: Goat anti-Ror1 polyclonal antibody used as primary antibody. The secondary antibody in all samples was FITC-conjugated anti-goat antibody
|
|