Nanosilver from Mangosteen Peel Extract (Garcinia mangostana L.) for Antibacterial Dental Preventive Agents
-
 Kurniawati Sugiaman , Vinna
Faculty of Dentistry, Maranatha Christian University, Bandung 40164, West Java, Indonesia, Tel: +62 8122115650; E-mail: vinnakurniawati@yahoo.co.id
Kurniawati Sugiaman , Vinna
Faculty of Dentistry, Maranatha Christian University, Bandung 40164, West Java, Indonesia, Tel: +62 8122115650; E-mail: vinnakurniawati@yahoo.co.id
-
Jeffrey , Jeffrey
-
Faculty of Dentistry, Universitas Jenderal Achmad Yani, Cimahi 40531, West Java, Indonesia
-
Widowati, Wahyu
-
Faculty of Medicine, Maranatha Christian University, Bandung 40164, West Java, Indonesia
-
Elisabeth, Mariska
-
Faculty of Medicine, Maranatha Christian University, Bandung 40164, West Java, Indonesia
-
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung 40163, West Java, Indonesia
-
Ayuni , Vini
-
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung 40163, West Java, Indonesia
-
Septyawan Hadiprasetyo , Dhanar
-
Biomolecular and Biomedical Research Center, Aretha Medika Utama, Bandung 40163, West Java, Indonesia
-
Faculty of Pharmacy, Universitas Jenderal Achmad Yani, Cimahi 40531, West Java, Indonesia
Abstract: Background: Teeth are vital structures prone to issues such as caries and plaque formation, often caused by Streptococcus mutans (S. mutans). This issue can be mitigated using natural ingredients like mangosteen fruit (Garcinia mangostana L.), especially its peel, is known for its medicinal benefits. However, its extract may take time to show effects, so it is being combined with nanosilver for improved drug distribution. To observe the antibacterial and antibiofilm potential of Mangosteen Peel Extract (MPE) in nanosilver form as a preventive agent in dentistry.
Methods: The extraction was succeeded by a phytochemical assay and biosynthesis of MPE into Mangosteen Peel Extract Nanosilver (MPNs). Particle Size Analysis (PSA) and Transmission Electron Microscopy (TEM) were used to study this procedure. Disc diffusion tests were used to evaluate the antibacterial properties, and the Minimum Inhibition Concentration (MIC) and Minimum Bactericidal Concentration (MBC) were also determined. Furthermore, the antibiofilm activity against S. mutans was investigated.
Results: the phytochemical contents in MPE were flavonoids, tannins, saponins, phenols, alkaloids, triterpenoids, and terpenoids. Particle size of MPNs was 126.1 nm and the Polydispersity Index (PDI) was 0.419. The highest antibacterial concentration as inhibition zone against S. mutans was 16.37±0.38 mm and 119.37±2.16% inhibitory activity, at the highest concentration (100%) p<0.05. The percentage of biofilm inhibition against S. mutans was 27.64-105.94% which was concentration dependent.
Conclusion: MPNs has potential as an antibacterial and antibiofilm agent that can be used as a preventive agent in medicine.
Introduction :
Teeth, body structures that encase soft tissue, must be maintained to avoid dental problems 1. In Indonesia, oral health prevalence is 57.6%, with a DMF-t score of 7.1%. Children aged 5-9 years experience 54% tooth decay or cavities and 92.6% experience caries 2. Caries forms due to demineralization and remineralization 3. Caries caused by Streptococcus mutans (S. mutans), a predominant bacterium responsible for caries that can generate organic acids 4. These acids erode the mineral layer on the enamel surface, leading to the formation of dental caries if continuous 5.
Antimicrobial medicines like antibiotics can inhibit oral bacteria growth but can lead to increased resistance 6. The component antibiotics can cause tooth discoloration and dentin demineralization 7. Herbal product was preferred over chemical drug due to minimal effect and affordable price, mangosteen peels for example 8. The peel is rich in phytochemical compounds that have anti-inflammation 9, antibacterial, and antifungal properties 10. To enhance the absorption of these compounds, nanoparticles have been formulated to regulate particle size, surface properties, and release active substances 11. Nanosilver, derived from Fomes fomentarius and Gardenia jasmonides, has shown successful effects in dentistry, including the use of silver nanoparticles (AgNs) in dental implants 12-14. Advances in nanoparticle-based dental treatments have to date been produced in the form of commercialized products, but these products are still limited such as dental adhesives (NanoCare Gold DNT™) 15, sealers (GuttaFlow™ Coltène-Whaledent) 16,17, In dentistry, advances in silver nanoparticles are used in conjunction with composites, such as Chitalac-Ag 18, AgNP-methyl polymethylmethacrylate 19, amorphous calcium AgNP-phosphate 20, and fluoride (Nano Silver Fluoride) 21. However, the products are still limited and still use chemical methods that use chemicals and expensive production costs 22.
Until now, there have not been many reports related to the advantages of nanosilver derived from MPE as a treatment for dental problems. Thus, this research seeks to establish the potential of mangosteen peel extract nanosilver (MPNs) through evaluation of active compound identification, followed by nanosilver characterization with Particle Size Analyzer (PSA), Transmission Electron Microscopy (TEM), antibacterial evaluation using disc diffusion, Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC), and antibiofilm tests against S. mutans bacteria that cause dental health damage.
Materials and Methods :
Phytochemical assay of MPE: The MPE was processed by PT FAST, Depok (CoA 00106202075) with the maceration method using solvents and excipients in the form of deionized water. Phytochemical screening of MPE was evaluated as adapted from Widowati et al 23 and Prahastuti et al 24 to qualitatively detect flavonoids, tannins, phenols, steroids/triterpenoids, saponins, terpenoids, and alkaloids.
Flavonoids: A test tube containing around 10 mg of each extract was then filled with Mg (Merck; 1058151000) and 2N HCl. After being heated for five to ten min, the mixture was cooled and filtered. Next, the filter was mixed with a solution of amyl alcohol. The creation of a red or orange hue indicated a favorable response 23,24.
Phenols: On a drip plate, around 10 mg of each extract was added, and the mixture was then mixed with 1% FeCl3 (Merck 1.03861.0250, USA). The color indicated the existence of phenols 23,24.
Steroids/triterpenoids: The drip plate was coated with acetic acid after 10 mg of each extract was added. One drop of strong H₂SO₄ was added to the sample after ten to fifteen min. Steroids were identified by the appearance of a green or blue color, while triterpenoids were indicated by the appearance of a red or orange precipitate 23,24.
Saponins: Approximately 10 mg of each extract was added to a test tube with water, boiled for 5 min, and then shaken vigorously. Foam on the surface of the solution signaled the presence of saponin 23,24.
Tannins: In a test tube, around 10 mg of each extract and 2 ml of 2N HCl were mixed, and the test tube was heated in a water bath for 30 min. The mixture was filtered after cooling down. After that, amyl alcohol was combined with the filtrate. When purple appears, it indicated a favorable response 23,24.
Terpenoids: A drip plate was coated with around 10 mg of each extract, then vanillin (SIGMA, V1104-100G) and a solution of H2SO4 were added. Purple coloration indicated a favorable response 23,24.
Alkaloids: Each extract was weighed out to be around 10 mg and mixed with 10% ammonia in a test tube. Two liquid layers formed because of the addition of chloroform to the mixture. After being removed and placed in a fresh test tube, the lower layer underwent 1N HCl treatment until two separate layers emerged. After that, the top layer was moved to another test tube and one or two drops of Dragendorff solution were added. Positive results were demonstrated by the emergence of a yellow color 23,24.
MPNs biosynthesis: The preparation of 1 mM AgNO3 solution for biosynthesis of MNPs nanoparticles (AgNPs) began with dissolving 42.25 mg of AgNO3 (Merck, 1.01512.0100) powder in 250 ml of distilled water using chemical glassware. The solution was stirred until homogeneous to achieve a concentration of 1 mM AgNO3. Subsequently, biosynthesis of AgNPs was initiated by dissolving 0.2 g of MPE in 50 ml of 96% ethanol. The procedure followed a modified method, where the MPE was mixed with the 1 mM AgNO3 solution at a ratio of 1:9. Each mixture was agitated with a magnetic stirrer at 250 rpm for 30 min, at temperatures of 60°C, 70°C, and 80°C 25.
PSA, TEM analysis of MPNs: A dynamic light scattering particle size analyzer (Horiba SZ-100) was utilized to assess particle size. For analysis, a cleaned cuvette containing a 10 ml sample was put within the sample chamber 26,27. Furthermore, Transmission Electron Microscopy (TEM) (HT7700) was used to analyze the morphology of the MPNs at magnifications ranging from 10,000 to 500,000×.
Antibacterial and biofilm test: Antibacterial MPNs with Kirby-Bauer disk diffusion: Antimicrobial testing against S. mutans (ATCC® 25175™) involved cotton swabs soaked in bacterial suspensions, which were then evenly spread onto Mueller Hinton Agar (MHA) plates (HIMEDIA, M173-500G), followed by incubation at 37°C for 24 hr. Sterile paper disks (6 mm diameter) were impregnated with 20 µl of each test sample using a micropipette and allowed to dry under aseptic conditions at room temperature. The prepared disks were then placed on the inoculated MHA plates. Disks soaked in DMSO 5% (Merck, 1.029.521.000) and Chlorhexidine 0.2% served as negative and positive controls, respectively. Zones of inhibition were measured and averaged after incubation 28,29.
MIC, MBC activity of MPNs toward S. mutans: The next antimicrobial test began with prepared the inoculum for each bacterium and fungus using the direct colony suspension method before determined MIC and MBC levels. Inoculum was obtained by transferring S. mutans colonies grown on MHA for 24 hr into Mueller Hinton Broth (MHB) (HIMEDIA, M391-500G). The turbidity was adjusted to match the McFarland 0.5 standard solution (1.5×108 CFU/ml). MIC levels were determined using 96-well plates filled with various concentrations (100%, 75%, 50%, 25%, 12.5%, 6.25%, and 3.13%) of MPNs, supplemented with bacterial culture and incubated at 37°C for 24 hr. 100% MPNs concentration was a concentration without additional solvent, 75% MPNs was by taking 750 µl of MPNs added with 250 µl of 5% DMSO, for concentrations of 50%-3.13% using multilevel dilutions dissolved with 5% DMSO. Chlorhexidine 0.2% (Minosep) served as the positive control, while 5% DMSO was the negative control, and growth control contains bacteria or fungi with MHB. After incubation, turbidity was measured using spectrophotometry at a wavelength of 500-600 nm. MBC levels are determined by taking 100 µl of each MIC result, performing serial dilutions (102-105), and platted 50 µl of each dilution on MHA using the pour plate technique. Plates were then incubated at 37°C for 24 hr, and colony counts were performed using a colony counter (Funke Gerber 8500) 29,30.
Biofilm activity of MPNs: Biofilm assays were conducted following the protocol outlined by Stepanović et al 31, using Brain Heart Infusion Broth (BHI) (HIMEDIA, M120-500G). The bacterial suspension's turbidity was calibrated to approximately 0.5 McFarland standard (~108 CFU/ml). Subsequently, 50 μl of bacterial suspension was transferred into sterile 96-well plates containing 100 μl of MPNs. Negative controls consisted of 200 μl BHI, while positive controls included 0.2% chlorhexidine. The plates were incubated aerobically at 37°C without agitation. After 24 hr, the liquid from each well was carefully discarded, and the wells were gently washed three times with Phosphate-Buffered Saline (PBS, Biowest X0520-500, pH=7.2). The biofilm adhered to each well was stained with 125 μl of 0.1% crystal violet solution for 15 min at room temperature. Excess stain was eliminated by rinsing with distilled water. The microplates were then allowed to air-dry, and the dye attached to the biofilm cells was dissolved with 125 μl of 30% acetic acid per well. The Optical Density (OD) of the dissolved crystal violet was measured at 570 nm (OD570) using a microplate spectrophotometer reader (Multiskan Go, Thermo Scientific, 51119300) 29,31.
Data analysis: SPSS (version 20.0) was used to analyze the data, and the mean±standard deviation was used to present the findings. Levene’s and Shapiro–Wilk tests were used to verify homogeneity and normality, respectively. While regularly distributed data were examined using One-Way ANOVA and further investigated using the Tukey HSD Post-Hoc test, non-normally distributed data were evaluated using the Kruskal-Wallis and Mann-Whitney U tests. At p<0.05, statistical significance was established.
Results :
Phytochemical in MPE: According to the phytochemical test results shown in table 1, the extract of MPE contains flavonoids, tannins, saponins, phenols, alkaloids, triterpenoids, and terpenoids.
Characterization of MPNs: Based on table 2, the average particle size of MPNs was 126.1 nm. In addition, and Polydispersity Index (PDI) was 0.419. Morphologically MPNs observed under a TEM microscope (10000-500000×), with various magnification sizes, in figure 1, morphology observed MPNs shape produced was spherical.
Antibacterial MPNs by disk diffusion in S. mutans: Antibacterial testing using the disc diffusion method, MPNs had antimicrobial activity against S. mutans bacteria characterized by the presence of an inhibition zone or clear zone formed. The highest concentration of 100% MPNs (treatment IX) had anti-microbial activity with an inhibition zone diameter was 16.37 mm, while 3.125% MPNs (treatment III) had an inhibition zone diameter was 2.52 mm. Various concentrations of MPNs showed significant differences in inhibition zones (p<0.05) (Figure 2).
Antibacterial of MPNs by MIC& MBC evaluation in S. mutans: Figure 3 displays the viability and inhibition of S. mutans bacteria resulting from treatment with MPNs. Based on the findings, the MIC was observed in treatment VII (25% MPNs) with a percentage of 65.14%. While MBC was observed by treatment X (100% MPNs) with a percentage value of 119.37%. The MPNs treatment demonstrated both growth and inhibition of S. mutans. The higher concentration of MPNs lowered the growth rate and increased the inhibition of S. mutans. This data aligned with the number of colonies reported in (Table 3).
The effect of MPNs as antibiofilm: The results of biofilm inhibition are illustrated in figure 4, it showed that MPNs inhibited biofilm formation. The highest antibiofilm activity was in treatment X (100% MPNs) with the lowest antibiofilm effect was treatment V (6.25% MPNs), while treatment IV (3.125% MPNs). The antibiofilm activity of MPNs was concentration-dependent; higher concentrations increased biofilm inhibition.
Discussion :
Poor dental health can affect the chewing and digestive system 32, and the phytochemical content of Mangosteen Peel Extract (MPE) is crucial for maintaining oral health. The study found that MPE contains flavonoids, tannins, saponins, phenols, alkaloids, triterpenoids, and terpenoids, with total flavonoids measured at 17.66±0.19 μg/mg 33,34. Active compounds in MPE including α-mangostin, γ-mangostin, garcinone-C, garcinone-D, garcinone-E, gartanin, and smeathxanthone-A, which have high potential as antioxidants and anti-inflammation agents 35,36.
The study investigates the biosynthesis of nanoparticles (MPNs) with a size range and PDI value in table 2. The results showed that the distribution algorithm operated well, with PDI values observed in the range of 0.2–0.5, and an average PDI of 0.419. This value falls within the acceptable range for moderate dispersity. According to Danaei et al 37, a PDI <0.3 typically indicates monodisperse systems, while values up to 0.5 are still considered moderately polydisperse and acceptable for nanoparticle formulations, particularly in green synthesis systems where some heterogeneity is expected due to the natural variability in reducing agents 37. It is worth noting that the TEM figure obtained (Figure 1) was captured at an external laboratory due to the unavailability of TEM instrumentation in our facility. While the figure clarity may not be optimal, it still demonstrated the characteristic spherical morphology of the synthesized nanoparticles, supporting the size and distribution data obtained from PSA. This limitation is acknowledged as a constraint in figure optimization for nanomaterial studies. In figure 1 and the sizes listed in table 2 correlate with nanosilver properties, which are significantly influenced by shape and size, with non-uniform size obtained due to van der Waals force promoting aggregation 38. Silver nanoparticles can be chemically synthesized (AgNPs) through various methods. The most common method to synthesize silver nanoparticles is the chemical reduction of silver nitrate (AgNO₃) using reducing agents such as sodium borohydride (NaBH₄), ethylene glycol, or citrate 39. The addition of such materials serves as a booster of their monodispersity 40. However, a study by Mousavi-Khattat et al 41 showed that although chemically synthesized silver nanoparticles have higher stability after synthesis, they tend to experience a faster decrease in stability compared to green-synthesized nanoparticles 41. On the other hand, chemically synthesized products are subject to agglomeration and precipitation over time, which reduces their effectiveness in applications, especially in the antimicrobial context 22. The use of many tools and chemicals also increases production costs and may pollute the environment and pose risks to human health 42. This discussion of chemical synthesis serves not only as contextual background but also as a rationale for selecting a green synthesis method using mangosteen peel extract in our study. By outlining the limitations of conventional chemical approaches, we highlight the significance of adopting a more environmentally sustainable, less toxic, and economically feasible biosynthesis strategy.
Plant, bacterial, and fungal extracts can be used as reducing and stabilizing agents, which minimize the use of toxic chemicals commonly used in chemical synthesis 43. Ahmed and Mustafa also noted that plant-mediated synthesis produces nanoparticles that are less likely to elicit toxic responses in living organisms, making them suitable for pharmaceutical applications 44. In addition, Rani 45 also confirmed that green-synthesized nanoparticles showed lower cytotoxicity to human cells compared to chemically synthesized ones 45. The result demonstrated that stability and toxicity are inseparable from the phytochemical content present in plants, which can enhance the antibacterial efficacy of nanoparticles, making them more effective against a wider range of pathogens 46. Procedurally, the biosynthesis of nanoparticles with natural extracts has fewer steps and can be carried out at ambient temperatures, making them more accessible for various applications 47. Moreover, the scalability of green synthesis is promising, as it can be adapted for larger production volumes without significant changes to the methodology 48.
In comparison with existing silver nanoparticle formulations in dentistry, and previous studies that have developed silver nanoparticles with various synthesis methods and applications to improve effectiveness in antibacterial activity, or support in dental treatment, such as silver nanoparticles incorporated with Glass Ionomer Cement (GIC) for pediatric dental treatment have indeed been shown to maintain their anti-caries effects 49. However, despite its effectiveness, GIC dental treatment with silver nanoparticles has weak mechanical and physical properties, such as low fracture strength, reduced wear resistance, and a tendency to discolor over time 50,51. These will affect the effectiveness of nanoparticles in dental care. In our study, silver nanoparticles synthesized with mangosteen peel are relatively more environmentally friendly, affordable, and easily accessible 52. The cutting-edge use of silver nanoparticles was published in a previous study by Moaddabi et al 53, involving an in vivo rabbit model of wound healing and microbial evaluation using AgNPS resulting in good dental wound healing and reduced microbial drivers of oral disease 53. on the other hand, the research of Craciunescu et al 54 also reported that AgNPs was able to increase anti-inflammatory activity by showing the growth of new blood vessels in 3D construction of gingival fibroblasts as a model of oral lesions and in vivo on chicken embryo Chorioallantoic Membrane (CAM) to analyze angiogenesis 54.
In this study, the effect of nanosilver was concentration-dependent. Higher concentrations of nanosilver tended to produce larger particles and vice versa 55. S. mutans bacteria were observed using disc diffusion and MIC, and MPNs showed an increase in concentration causing significance of inhibition. High concentrations of MPNs can downregulate genes involved in biofilm production, potentially preventing the creation of extracellular polysaccharides essential for biofilm formation 56. Biofilms can form due to the presence of a community of microorganisms bound to the surface and surrounded by an Exopolysaccharide (EPS) matrix 57. Mangosteen silver nanoparticles have a complex mechanism in reducing biofilms. Silver nanoparticles produce AgNPs, which will generate Reactive Oxygen Species (ROS) when exposed to water or biological fluids, causing cellular damage, oxidative stress, and bacterial death 58. Ag+ promotes increased antimicrobial activity of nanosilver by damaging the bacterial cell membrane, causing cell contents to leak out, leading to cytoplasmic membrane damage and cell death 59. It also interacts with bacterial DNA, causing genetic damage, inhibiting replication and growth, and interfering with metabolic processes and DNA repair 60.
In addition to the presence of ROS activity, the interplay between Ag+, the phytochemical content of the extract, and the concentration tested is also involved in the process. Nalawati et al 61 found that the smaller size of synthesized nanosilver, compared to commercial nanosilver with a size difference of 33.8 nm and 44.8 nm, effectively inhibits bacteria. This is because smaller AgNPs have a greater surface area for interacting with bacteria than larger ones, so they can provide a greater antibacterial effect by releasing Ag+ in a sustainable manner 62. Nanosilver's antibacterial potential is attributed to its active compounds, such as xanthones, which can reduce S. mutans biofilm growth 62. These amphiphilic compounds, including α-mangostin, gartanin, γ-mangostin, garcinone B, and garcinone E, with bacterial cell membranes and cause disruption 63. At the highest concentration (100%), MPNs exhibited a biofilm inhibition percentage of 105.94%. This value slightly exceeds 100% due to the significantly lower OD570 readings of the treated samples compared to the negative and even blank controls. This extreme reduction in optical density indicates near-complete eradication or prevention of biofilm formation. The inhibition percentage was calculated using OD-based comparison, and when the OD of the treated sample is below the baseline, values >100% can result mathematically. Similar phenomena have been noted in other studies using potent nanoparticle-based systems against S. mutans, suggesting that such high inhibition values reflect strong antibiofilm efficacy rather than error in calculation 56,57. Therefore, we retained this value with clarification to emphasize the superior activity of MPNs. Studies using biomass and biochar containing hydroxyl groups have shown that these mangostin compounds effectively reduce Ag+ 64.
The novelty of this research lies in the application of MPE as a bio-reducing and stabilizing agent for the green synthesis of silver nanoparticles (MPNs), providing a sustainable and phytochemical-rich alternative to conventional chemical methods. While silver nanoparticles have been extensively studied for dental applications, the incorporation of xanthone-containing plant extracts in the synthesis process remains limited. This study advances current knowledge by demonstrating that MPNs exhibit significant antibacterial and antibiofilm activities against S. mutans, with superior performance at higher concentrations. Furthermore, the eco-friendly and cost-effective synthesis approach enhances the translational potential of MPNs for future development in oral healthcare, particularly in settings with limited access to advanced manufacturing infrastructure. This study provides promising results regarding the potential of Mangosteen Peel Extract Nanosilver (MPNs) as an antibacterial agent.
However, there are some limitations that should be noted. Human clinical trials have not been conducted, so the effectiveness of MPNs in a clinical setting has not been verified. The particle size distribution has not been uniform, which may affect antibacterial activity and product stability. This study was only limited to the antibacterial effect in the oral cavity, so further evaluation regarding toxicity and biocompatibility is needed. It has been suggested by Recordati et al 65 that silver nanoparticles exhibit size-dependent toxicity, with smaller particles (15-20 nm) showing significant accumulation in various organs, leading to potential side effects 65. This a consideration for future research. This study has several limitations that should be acknowledged. The antibacterial and antibiofilm assessments were limited to S. mutans, a primary etiological agent of dental caries, without evaluating potential interactions with other oral pathogens involved in polymicrobial infections. The cytotoxicity of MPNs was not evaluated, which is essential for assessing their safety in biomedical applications. The stability study (short-term or accelerated) was not conducted to determine the physical and functional stability of the nanosilver formulation. These limitations highlight the need for further research to expand the antimicrobial spectrum tested, evaluate cytocompatibility using relevant cell lines, and perform stability testing to ensure consistent efficacy and safety. Future studies should also focus on optimizing the synthesis process to improve particle size uniformity and reproducibility.
Conclusion :
This study demonstrates that MPNs are rich in phytochemicals and possess antibacterial and antibiofilm activity against S. mutans, suggesting their potential as preventive agents in dental care. However, the cytotoxic effects on human oral cells were not assessed and should be addressed in future studies through in vitro cytocompatibility testing.
Acknowledgement :
We are also thankful to Maranatha Christian University in the internal funding research Scopus output scheme without partner collaboration with a research grant number of 023/SK/ADD/UKM/V/2024 as a source funding. Thanks to PT FAST for providing BPOM standardized MPE, and AMUBBRC team for facilitating and supporting this research.
Funding: The research is funded by Maranatha Christian University, Bandung, West Java, Indonesia the internal funding research Scopus output scheme without partner collaboration with a research grant number of 023/SK/ADD/UKM/V/2024.
Ethical approval: This study did not involve human or animal subjects; therefore, no ethical approval was required.
Conflict of Interest :
The authors declare that there is no conflict of interest. The authors alone are responsible for the accuracy and integrity of the paper content.
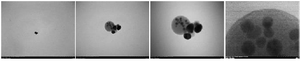
Figure 1. MPNs morphology under TEM magnification 10000×, 50000×, 100000× and 500000×.
|
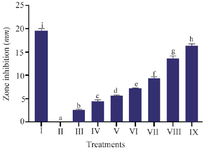
Figure 2. The effect of various MPNs concentration on S. mutans toward zone inhibition.
* Data are presented as mean±standard deviation. For each treatment, the test was performed in three replicates. Different superscripts (a, b, c, d, e, f, g, h and i) indicate significant differences between different MPNs treatments (p<0.05, Independent T-test). Roman numeral I: Positive control chlorhexidine 0.2%, II: Negative control DMSO, III-IX are various concentrations of MPNs treatments (3.125, 6.25, 12.5, 25, 50, 75, 100%).
|
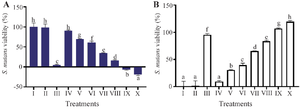
Figure 3. The effect of various MPNs concentration toward viability, inhibition of S. mutans.
* Data are presented as mean±standard deviation. For each treatment, the test was performed in three replicates. Different superscripts (a, b, c, d, e, f, g, h) indicate significant differences among different MPNs treatments toward S. mutans viability and inhibition (p<0.05, Independent T-test). Roman numeral I: Growth control; II: Negative control DMSO III: Positive control chlorhexidine 0.2%, IV-IX are various of MPNs treatments (3.125, 6.25, 12.5, 25, 50, 75, 100%).
|
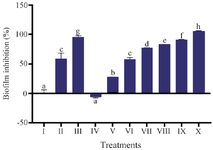
Figure 4. The effect various MPNs concentration toward biofilm inhibition of S. mutans
* Data are presented as mean±standard deviation. For each treatment, the test was performed in three replicates. Different superscripts (a, b, c, d, e, f, g, h) indicate significant differences among MPNs treatments (p<0.05, Independent T test). Roman numeral I: Growth control; II: Negative control DMSO III: Positive control chlorhexidine 0.2%, IV-IX are variations of MPNs treatments (3.125, 6.25, 12.5, 25, 50, 75, 100%).
|
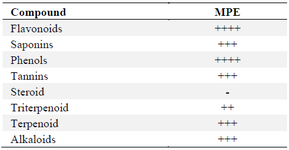
Table 1. Phytochemical assay of MPE
* The data of phytochemicals content in MPE are presented in qualitative data, which ++++ (very high content); +++ (high content); ++ (moderate content); + (less content); - (not detected).
|
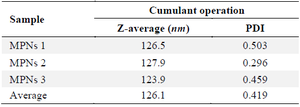
Table 2. Particle size results of MPNs by PSA
Measurements of PSA were done in three repetitions.
|
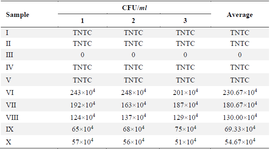
Table 3. The effect various MPNs concentration toward S. mutans colonies number
TNTC: Too Numerous to count >250. Roman numeral I: Growth control, II: Negative control, DMSO III: Positive control chlorhexidine 0.2%, IV-IX are various of MPNS treatments (3.125, 6.25, 12.5, 25, 50, 75, 100%), measurements were done in three repetitions.
|
|