Challenges and Future Prospects of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) in Nanomedicine: A Focus on Toxicity, Imaging, and Theranostics
-
Alvandi, Maryam
-
Cardiovascular Research Center, Hamadan University of Medical Sciences, Hamadan, Iran
-
Department of Nuclear Medicine and Molecular Imaging, School of Medicine, Hamadan University of Medical Sciences, Hamadan, Iran
-
Nosrati, Sahar
-
Institute of Nuclear Chemistry and Technology, Dorodna 16 Str, 03-195, Warsaw, Poland
-
Mansouri, Ramin
-
Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
-
 Shaghaghi, Zahra
Cancer Research Center, Institute of Cancer, Avicenna Health Research Institute, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 81 38381737, Fax: +98 81 38381686 E-mail: z.shaghaghi90@yahoo.com, z.shaghaghi@umsha.ac.ir
Shaghaghi, Zahra
Cancer Research Center, Institute of Cancer, Avicenna Health Research Institute, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 81 38381737, Fax: +98 81 38381686 E-mail: z.shaghaghi90@yahoo.com, z.shaghaghi@umsha.ac.ir
-
Saednia, Shahnaz
-
Farabi Hospital, Isfahan University of Medical Sciences, Isfahan, Iran
-
Alipour, Sara
-
Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
-
Fathi, Farzad
-
Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran
Abstract: Superparamagnetic Iron Oxide Nanoparticles (SPIO Ns) have emerged as a pivotal tool in nanomedicine, offering potential in drug delivery, imaging, and targeted therapies. However, their application is challenged by issues such as cytotoxicity, uneven biodistribution, and biocompatibility. SPIONs are predominantly cleared through renal or hepatobiliary pathways, with size and charge playing critical roles in determining their fate. While smaller SPIONs optimize renal clearance, their propensity to agglomerate and activate macrophages may induce inflammatory responses. Radiolabeled SPIONs face additional challenges in molecular imaging and nuclear medicine. Emerging strategies, such as chelator-free radiolabeling and multi-component nanoparticles, aim to address these limitations by improving targeting specificity and enhancing biocompatibility. Looking forward, SPIONs hold immense potential in theranostics, particularly in integrating imaging with targeted drug delivery and therapies. Advances in synthesis and surface functionalization may enhance their safety and effectiveness. Future research should focus on optimizing SPIONs, integrating them with therapeutic agents, and improving targeting and clearance mechanisms. Collaboration among experts and the use of Artificial Intelligence (AI) modeling could accelerate their development for personalized treatment applications. This review uniquely highlights recent advances in radiolabeled SPIONs for molecular imaging and targeted therapy, addressing challenges like biocompatibility, stability, and translational applicability.
Introduction :
Cancer remains a major challenge in medicine, accounting for a significant portion of global mortality 1. A crucial factor in addressing cancer effectively is early and precise diagnosis 2. Nanotechnology has emerged as a transformative tool in cancer management, offering advanced capabilities for early detection, precise identification, and personalized therapy 3,4. Among the most promising nanomaterials, Superparamagnetic Iron Oxide Nanoparticles (SPIONs) have gained considerable attention for their applications in diagnosis and therapy. These particles, typically composed of γ-Fe2O3 (maghemite) or Fe3O4 (magnetite), have been investigated as Magnetic Resonance Imag-ing (MRI) contrast agents, drug carriers, and mediators of hyperthermia. Current research on SPIONs is expanding their potential applications in diagnostic imaging as well as in targeted drug delivery systems. The integration of anticancer drugs with functionalized
SPIONs for site-specific therapy is a rapidly advancing field in cancer treatment. Radiolabeling extends their utility to Positron Emission Tomography (PET) and Single-Photon Emission Computed Tomography (SPECT), enabling sensitive whole-body molecular imaging with anatomical MRI precision 5,6. The clinical translation of radiolabeled SPIONs faces several challenges, including standardization of synthesis protocols, immunogenicity, and potential long-term toxicity. Unlike prior reviews that separately examine SPIONs or radiolabeled nanoparticles, this work provides an integrated perspective on radiolabeled SPIONs, assessing their synthesis strategies, surface modifications, biological interactions, and the translational barriers to clinical implementation in cancer theranostics.
Advances in functionalized MNPs for cancer therapy: Functionalized MNPs (Magnetic nanoparticles) are gaining traction for cancer therapeutics, with many in preclinical and early clinical development 7,8. Multifunctional MNPs have been engineered for targeted delivery of therapeutic agents such as small molecules and miRNAs, effectively addressing chemoresistance in cancer cells 9. For instance: Composite Nanoparticles: Magnetic iron oxide (Fe₃O₄) nanoparticles functionalized with β-cyclodextrin crosslinked with ethylenediaminetetraacetic acid (EDTA) or trastuzumab have shown excellent cellular uptake, tumor targeting, and therapeutic outcomes, including reduced cancer cell viability, apoptosis induction, and inhibition of proliferation in in vitro cancer models 10. SPION Functionalization: SPIONs with cores of Fe₃O₄, γ-Fe₂O₃, or other materials like nickel and cobalt have been modified with hydrophilic organic polymers [e.g., polysaccharides, dextran, Polyethylene Glycol (PEG), Polyvinyl Alcohol (PVA)] or targeting ligands (e.g., avidin, biotin, carboxyl groups). These modifications enhance biocompatibility, bio-distribution, and therapeutic efficacy by shielding the nanoparticles from biological degradation and improving their interaction with cancer tissues 7. The surface characteristics and oxidation state of SPIONs significantly influence their morphology, surface charge, and behavior in biological systems. For instance, surface charge impacts opsonization, a process where plasma proteins and biomolecules adhere to the nanoparticle surface, forming a protein corona that facilitates phagocytosis by macrophages. Studies have shown that smaller nanoparticle sizes and increased hydrophobicity can reduce opsonization, thereby improving circulation time 11. SPIONs’ surface properties, are such as crystallinity, roughness, and hydrophilicity, also play a crucial role in their in vivo applications, determining factors like blood circulation half-life and overall efficacy. These properties dictate the choice of coating materials, which may bond to the SPION surface through physical interactions or direct chemical binding.
A wide variety of materials are used to coat SPIONs, including: Polymers: PEG, Polylactic Acid (PLA), chitosan, dextran, and poly (lactic-co-glycolic acid) (PLGA); Biological molecules: Human serum albumin, mannose, alginate, and heparin; other functional coatings: Liposomes, arabinogalactan, and Polyethylene Imine (PEI). Coating materials such as PEG and dextran help prevent opsonization, reduce uptake by macrophages and the Reticuloendothelial System (RES), and mitigate aggregation. Conversely, uncoated SPIONs with hydrophobic surfaces exhibit rapid uptake by macrophages and accumulation in RES organs such as the liver, spleen, and lymph nodes 12,13. Additionally, coating SPIONs with liposomes can prevent aggregation and facilitate tumor cell targeting. These coatings enhance the blood half-life, colloidal stability, biocompatibility, and water solubility of SPIONs. They also improve accumulation at tumor sites via the Enhanced Permeability and Retention (EPR) effect, making them ideal for imaging and therapeutic applications. Furthermore, bio conjugation of biological moieties on SPION surfaces enables functional imaging modalities and theranostic applications 12,13.
Old-school ways of tagging SPIONs with radioactive markers usually leaned on chelators like Dodecane Tetraacetic Acid (DOTA) and Diethylenetriaminepentaacetic Acid (DTPA). But lately, new tricks skipping the chelators altogether have stepped up the game—boosting stability, cutting down on stray effects, and making them stick around longer in the body. By tapping into SPIONs’ built-in knack for grabbing metals, scientists can now slap radionuclides like 68Ga straight onto them. This tweak sharpens up how they move through the body and amps up imaging precision, raising the bar for early-stage lab models 14.
SPION characteristics and superparamagnetic properties
SPIONs are widely used in theranostics due to their unique physical and magnetic properties. Key characteristics that enable their biomedical applications include:
Size control: SPIONs range from 1–100 nm, making them comparable in size to biological entities such as cells (10–100 μm), viruses (20–450 nm), proteins (5–50 nm), and genes (2 nm wide, 10–100 nm long). Their size can be precisely tuned for specific applications 15,16. For intravenous applications, SPIONs with diameters between 10 and 100 nm are ideal; particles smaller than 10 nm or larger than 200 nm are rapidly cleared by the RES 17,18.
Superparamagnetic properties: SPIONs exhibit superparamagnetism, becoming magnetized only in the presence of an external magnetic field and rapidly losing magnetization once the field is removed. This property allows, precise control and localization of SPIONs in specific body regions under a magnetic field. Prevention of aggregation and embolization in capillary vessels during in vivo applications. Use in diverse applications such as molecular labeling, bio sensing, biomolecule separation, and targeted drug and gene delivery 19,20.
Localized heating for hyperthermia therapy: SPIONs can generate localized heat (45-47°C) when exposed to an alternating magnetic field, making them useful for hyperthermia therapy to selectively kill cancer cells.
Magnetic susceptibility: SPIONs' Superparamagnetic properties enhance magnetic susceptibility, which diphase protons in external magnetic fields. This makes SPIONs highly effective as contrast agents in MRI 15,21.
SPION synthesis: SPIONs have gained significant attention as contrast agents in MRI due to their ability to alter nuclear spin relaxation of water protons, enhancing the visibility of target regions by creating darkened contrast areas. These nanoparticles offer several advantages, including cost effectiveness, biodegradability, chemical stability, low toxicity, and tunable properties such as size, surface chemistry, and diffusion capacity. These attributes influence their biodistribution, metabolic pathways, and stability in biological systems 3,11,22. SPIONs are utilized in multimodal imaging for applications such as cell targeting, drug delivery, disease diagnosis, and treatment monitoring, potentially reducing imaging sessions and improving diagnostic efficiency. Some SPION formulations, such as Ferridex I.V® (for liver and spleen imaging) and Combidex® (for lymph node metastasis imaging), have been clinically approved 23,24.
Targeted SPIONs have shown promise as MRI contrast agents for detecting: Overexpressed receptors on tumor cell surfaces, abnormal angiogenesis in the tumor microenvironment, Circulating Tumor Cells (CTCs) and soluble tumor markers 18,25,26. However, SPIONs lack inherent radioactive properties essential for PET. To overcome this, SPIONs have been radiolabeled with isotopes such as 99mTc, 125I, 111In, 131I, 18F, 11C, 67,68Ga, 64Cu, and 124I. These radiolabeled SPIONs can be used in hybrid imaging techniques such as PET/MRI and SPECT/MRI, improving early detection, multimodal imaging, and theranostic capabilities 27,28. SPION synthesis can involve physical and biological methods, though these approaches face challenges such as scaling up production and achieving well-defined structures with consistent size distributions.
Major issues in SPION synthesis include:
Achieving mono-disparity to prevent aggregation and continuous nanoparticle growth caused by magnetic interactions and surface energy, controlling size, shape, and composition, optimizing magnetic properties for biomedical applications 13,26.
Imaging properties of SPIONs: Noninvasive imaging techniques are increasingly recognized for their ability to provide early diagnosis, whole-body coverage, and repeated assessments, surpassing the limitations of invasive biopsy techniques. While techniques like SPECT and PET are valuable, MRI remains the gold standard for noninvasive diagnostic imaging due to its high spatial resolution and detailed anatomical information (Table 1) 29. MRI contrast is categorized into T1-weighted (longitudinal relaxation) and T2-weighted (transverse relaxation) images, based on the relaxation processes involved 30. To achieve high-resolution molecular imaging using MRI, several factors must be addressed: Access to high-affinity probes with favorable pharmacodynamics. Probes capable of overcoming biological delivery barriers such as cell membranes, interstitial spaces, and vascular walls. Utilization of advanced biological or chemical strategies to enhance probe delivery and retention 31,32. MRI sensitivity relies on the relaxivity values of contrast agents. SPIONs are particularly effective as MRI contrast agents because they have high r2 relaxivities, which can exceed 200 mM⁻¹s⁻¹. This characteristic makes them especially suitable for T2-weighted imaging. In contrast, commercial T1 contrast agents consist of paramagnetic complexes made from transition or lanthanide metals, such as Gd³⁺ and Mn²⁺. However, these T1 agents often exhibit low sensitivity for specific imaging applications. As a result, significant research has been conducted into alternatives like SPIONs, which are clinically approved as T2 contrast agents for disease detection when used in conjunction with an external magnetic field 33. Relaxivity measurements also correlate with SPION accumulation in tumors, providing valuable diagnostic insights 34,35. The mechanism involves SPIONs interacting with surrounding water molecules to increase the relaxation rates of water protons. After exposure to a Radiofrequency (RF) pulse, protons return to equilibrium via longitudinal (T1) and transverse (T2) relaxation. SPIONs' superparamagnetic properties enhance the dephasing of water protons, which contributes to the contrast in MRI 36-38. SPIONs are primarily used as negative (T2) contrast agents in MRI due to their high magnetic susceptibility. However, smaller SPIONs have also demonstrated potential as positive (T1) contrast agents, further expanding their applications (Figure 1).
Toxicity and modification of SPIONs: The use of SPIONs in biomedical applications raises safety concerns, particularly regarding their cytotoxic potential. This toxicity often stems from oxidative stress triggered by the release of free iron ions (Fe²⁺) during SPION degradation within acidic cellular environments. Following phagocytosis and subsequent lysosomal degradation, free Fe²⁺ is released, which can catalyze the production of Reactive Oxygen Species (ROS) through the Fenton reaction. Elevated ROS levels can result in DNA damage, lipid peroxidation, and inflammatory responses, compromising cellular integrity 39,40. Under normal physiological conditions, ROS production is regulated by antioxidant systems such as Glutathione (GSH) and antioxidant enzymes. However, when oxidative stress overwhelms these systems, cells may experience cytotoxic effects mediated by signaling pathways like the Mitogen-Activated Protein Kinase (MAPK) and nuclear factor-kB (NF-κB) cascades. Low oxidative stress levels activate nuclear factor erythroid 2–related factor 2 (Nrf2), which promotes the expression of antioxidant and detoxification enzymes. In contrast, higher oxidative stress levels can exacerbate inflammation and cellular damage 41.
Cells manage iron homeostasis through proteins such as Divalent Metal Transporter 1 (DMT1), which imports iron, transferrin receptor (CD71), which facilitates transferrin-mediated iron uptake, and ferritin, which sequesters iron to prevent free radical formation. Iron export is regulated by ferroportin (FPN) and ZRT/IRE-like proteins. However, SPION degradation bypasses these regulatory mechanisms by releasing iron directly into the labile iron pool, potentially overloading these systems 42,43. To evaluate SPION-induced cytotoxicity, researchers measure biomarkers of oxidative stress, including lipid peroxidation, reduced glutathione, and antioxidant enzyme activity (e.g., catalase, glutathione peroxidase, and superoxide dismutase). Studies have shown that free iron from SPION degradation is the primary contributor to oxidative stress. Using iron chelators has been effective in mitigating ROS generation, Lactate Dehydrogenase (LDH) release, and DNA damage 44,45. Additionally, macrophage type influences SPION toxicity, with mouse alveolar macrophages exhibiting lower oxidative activity compared to peritoneal macrophages 44-46.
Unique size and shape-dependent toxicity: The small size and high surface area of SPIONs can amplify cytotoxic effects. Smaller SPIONs exhibit increased reactivity and cytotoxicity per unit mass compared to larger nanoparticles. However, nanoparticle shape also plays a role; for example, rod-shaped SPIONs have demonstrated greater toxicity than spherical ones, despite their smaller surface area. These variations suggest that factors beyond surface area, such as surface charge, composition, and shape, influence SPION toxicity 47,48. The pH of the surrounding environment and SPION surface properties, such as coatings and charge, also affect their cytotoxicity and biological interactions. For instance, uncoated SPIONs have been shown to decrease cell viability and increase ROS production in human fibroblasts at concentrations as low as 2 µg/cm2, with higher concentrations causing DNA damage 49-51. Surface modifications can mitigate these effects by altering SPION interactions with cells and proteins. By tailoring SPION size, charge, and surface coatings, researchers can reduce phagocytosis and protein adsorption, which influence clearance, biodistribution, and cytotoxicity. When SPIONs bind to serum proteins or form complexes with other biomolecules, their hydrodynamic size increases, reducing renal clearance and extending their circulation time. These complexes may also enhance tissue penetration and intracellular uptake, potentially leading to unintended cytotoxic effects 49,51. To minimize toxicity, strategies such as functionalizing SPION surfaces with biocompatible coatings or modifying their physicochemical properties have been employed. These approaches reduce ROS generation, protein adsorption, and oxidative damage, improving the safety profile of SPIONs for biomedical applications.
Site-specific accumulation: SPIONs are primarily excreted through either the renal or hepatobiliary pathways. Due to the potential for greater cytotoxicity from intracellular enzymatic modification in the hepatobiliary system, the renal pathway is generally preferred to minimize toxicity 51. For SPIONs to be excreted via the renal system, they must be small enough to pass through the glomerular filtration barrier, which has a physiological pore size of about 4.5-5 nm in diameter. Particles smaller than 4.5 nm can easily pass through, while particles ranging from 6–8 nm, especially if cationic, may still pass through these pores 52. However, caution is needed, as overly cationic or anionic particles can increase Hydrodynamic Diameter (HD) due to charge-induced adsorption by serum proteins 53. SPIONs do not distribute evenly throughout the body and tend to accumulate preferentially in certain organs. For optimal blood circulation during imaging, SPIONs should be sized between 10 nm and 200 nm. This size range helps avoid rapid clearance by the kidneys and prevents sequestration in the spleen 54. However, this size range can complicate the even distribution of SPIONs and potentially lead to toxicity. Due to their small size and large surface area, SPIONs tend to agglomerate before being phagocytized by macrophages in the liver (Kupffer cells) and other cells of the RES. This phagocytosis activates macrophages, triggering an inflammatory response that can involve the recruitment of various immune cells and the generation of ROS to destroy the foreign particles. Macrophages, once activated, may migrate throughout the body and differentiate into specific types such as Kupffer cells in the liver, osteoclasts in bone, microglia in the brain, alveolar macrophages in the lungs, and mesangial cells in the kidneys 55.
Tumor imaging and the growing potential of SPIONs: SPIONs are shaping up to be a real game-changer for spotting tumors in MRI scans. Lately, researchers have been digging into ultrafine SPIONs, which sneak into tumors more effectively by riding the wave of something called the EPR effect—basically, taking advantage of leaky tumor blood vessels. They’ve been a big help in imaging the liver and spleen, making it easier to tell apart different kinds of lesions. Sure, some SPION-based contrast agents got pulled off the shelves because they struggled to pick out liver lesions clearly, but they’re still a solid pick for folks with kidney issues. When it comes to brain tumors, SPIONs shine by helping measure blood volume in the region, giving doctors a clearer picture. Cancer’s a huge focus—understandably, given how much it matters worldwide—but SPIONs aren’t just a one-trick pony. Their strengths could stretch into all sorts of medical areas, opening up a ton of possibilities 56. Clinical studies have shown that ferumoxytol-boosted MRI is an ace at finding metastatic lymph nodes, hitting sensitivity rates over 90%, way better than the usual contrast agents. Plus, early Phase I trials testing SPIONs for magnetic hyperthermia—where they heat up to zap tumors—look promising for shrinking glioblastoma and prostate cancer. And then there’s the latest combo trick: pairing radiolabeled SPIONs with dual PET/MRI imaging, which is sharpening up diagnostics for heart conditions and cancer alike. On top of that, these magnetic nanoparticles are easy to tweak, which makes them perfect for cooking up blends that work across multiple imaging styles—like CT, PET/SPECT, ultrasound, fluoroscopy, or even photoacoustic imaging.
Challenges and functionalization: Despite their advantages, SPIONs face challenges like agglomeration and low affinity for biomolecules.
Agglomeration: This can lead to loss of functionality and in vivo complications. Coating SPIONs with biocompatible materials solves this issue, improving colloidal stability.
Low affinity for biomolecules: Functionalizing SPION coatings with specific ligands and biological molecules enhances their affinity for targeted biomolecules and therapeutic agents 57,58.
Imaging issues: One big roadblock stopping SPIONs from hitting the clinic is that making and tweaking them isn’t always consistent. Differences in how they’re cooked up can mess with their physical and chemical traits, which in turn throws off how well they work for imaging.
To sort this out, regulatory folks and nanomedicine groups are teaming up to set some ground rules—think standard playbooks for controlling their size spread, surface charge, and stability checks 59. By addressing these challenges, SPIONs continue to hold promise for advancing theranostics, particularly in imaging, targeted drug delivery, and cancer therapy.
Conclusion :
SPIONs have significant potential in nanomedicine for imaging and targeted drug delivery. However, issues like cytotoxicity, uneven distribution, and safety concerns limit their clinical use. Advances in synthesis and functionalization aim to improve their biocompatibility and efficacy. By integrating SPIONs with therapeutic agents, localized treatments with fewer side effects could be achieved. Ongoing research is focused on addressing these challenges, refining SPION designs, and utilizing AI to optimize their medical applications.
Acknowledgement :
The authors would like to thank the Clinical Research Development Unit of Farshchian Heart Hospital, Hamadan University of Medical Sciences, Hamadan, Iran, for their assistance throughout the period of study.
Funding: None.
Conflict of Interest :
Authors declare no conflict of interest.
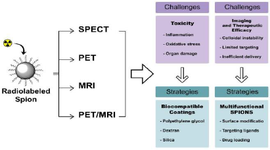
Figure 1. Schematic figure to visually organize the discussed challenges and proposed strategies.
|

Table 1. Comparison of imaging modalities used with radiolabeled SPIONs
|
|