Short-Term Inflammatory Exposure Affects Umbilical Cord-derived Mesenchymal Stem Cells Migration and Differentiation Through Modulation of NLRP3 Inflammasome Expression
-
Junaidi, Helsy
-
Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
-
 Sukmawati , Dewi
Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, Tel: +62 21 3146129; E-mail: ds_histoui@outlook.com, dewi.sukmawati@ui.ac.id
Sukmawati , Dewi
Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia, Tel: +62 21 3146129; E-mail: ds_histoui@outlook.com, dewi.sukmawati@ui.ac.id
-
Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
-
Narayanan V , Anoop
-
Nitte (Deemed to be University), NGSM Institute of Pharmaceutical Sciences (NGSMIPS), Department of Pharmaceutics, Mangaluru, Karnataka, India
-
A. Pawitan , Jeanne
-
Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
-
Department of Histology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
-
Stem Cell and Tissue Engineering (SCTE) Research Cluster, Indonesian Medical Education and Research Institute (IMERI), Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
-
Integrated Service Unit of Stem Cell Medical Technology (IPT TK Sel Punca), Dr. Cipto Mangunkusumo General Hospital (RSCM), Jakarta, Indonesia
-
Jusman , Sri Widia
-
Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia
Abstract: Background: An innovative approach for tissue restoration using Umbilical Cord-derived Mesenchymal Stem Cells (UC-MSCs) is hindered by their poor survival rate due to the detrimental effects of the injured tissue microenvironment. Activation of NLRP3 inflammasome in an inflammatory environment which is followed by cellular impairment, has been reported. However, the expression of NLRP3 inflammasome in UC-MSCs in response to the inflammatory environment is not well understood. This study aims to investigate the impact of short-term exposure to an inflammatory environment induced by Lipopolysaccharide (LPS) on hUC-MSCs, focusing on cell viability, migration, differentiation, and the expression of NLRP3 inflammasome-related genes.
Methods: hUC-MSC were exposed to LPS at concentration of 10 and 50 μg/ml for 3 and 6 hr. Cell viability was assessed using CCK-8 assay, migration capacity was evaluated using a scratch test, and differentiation capacity and the expression of NLRP3 inflammasome-related genes were measured using qRT-PCR.
Results: Short-term LPS induction did not affect the viability of hUC-MSCs but reduced their migration and differentiation capacity, particularly at 50 μg/ml for both time points (p<0.05). The induction caused an increase in the mRNA levels of NLRP3, TLR-4, and RelA/p65, which correlated with elevated expression of caspase-1 and IL-1β.
Conclusion: Short-term exposure to LPS influences hUC-MSCs by upregulating NLRP3, TLR4/ReIA (p65), IL-1β, and caspase-1 mRNA levels, leading to impaired migration and differentiation ability. This study underscores the significant impact of short-term exposure to an inflammatory microenvironment on hUC-MSC, potentially compromising their migration and differentiation capacity through the NLRP3 pathway.
Introduction :
Mesenchymal Stem Cells (MSC) possess superior properties such as broad differentiation potential, high proliferation capacity, paracrine activity, and immunomodulatory abilities 1. However, when administered in the context of injured tissue, they face challenges due to the adverse microenvironment. This environment is characterized by high levels of inflammation and the presence of Reactive Oxygen Species (ROS) as part of the body's healing response 2. These conditions can lead to reduced cell survival rates post-transplantation. Factors such as low oxygen levels, oxidative stress, and mechanical stress within the injured tissue microenvironment can promote the infiltration of inflammatory cells, leading to local inflammation 3. This inflammatory environment may have detrimental effects on transplanted stem cells, including delays or reductions in cell proliferation and migration, limitations in differentiation capacity, induction of senescence, increased apoptosis, and potential DNA damage 4-7.
Therefore, stem cell applications must prioritize methods and timing that ensure the applied cells modulate the microenvironment, increasing survival rates and initiating the healing process. When tissue is damaged, the microenvironment triggers a cascade of inflammatory responses, beginning with the release of Damage-Associated Molecular Patterns (DAMPs) by stressed, injured, or dying cells 8. These molecules activate the NLRP3 inflammasome, a key component of the innate immune response. The NLRP3 activation process involves the formation of the NLRP3 inflammasome complex, which can impact cell behaviour 8,9. Ahn et al revealed that the activation of the NLRP3 inflammasome pathway hindered the differentiation of MSCs into osteogenic and adipogenic processes, which were reversed upon blocking the expression of proteins involved in inflammasome assembly 10. NLRP3 is known to be involved in cell transcriptional regulation, inflammation, and immunological modulation 10,11. However, importantly, its effects may vary in MSCs.
Gram-negative bacteria have cell walls containing Lipopolysaccharide (LPS), commonly used to induce inflammation or recreate an inflammatory microenvironment. LPS triggers inflammation and tissue injury through oxidative stress and inflammatory mechanisms, which can affect the microenvironment of stem cells. Inflammation-induced by LPS exposure can influence the viability, differentiation, and regenerative abilities of MSCs 12. The impact of inflammation on MSCs depends on the context and the duration of exposure. Understanding these effects is essential for developing effective therapies that use MSCs for tissue repair, wound healing, and inflammation 13. This study aims to investigate the impact of short-term inflammatory exposure using LPS induction, on human Umbilical Cord-derived Mesenchymal Stem Cells (hUC-MSCs), focusing on cell viability, migration, differentiation, and the expression of NLRP3 inflammasome-related genes.
Materials and Methods :
This in vitro study used mesenchymal stem cells from cryopreserved hUC-MSCs obtained from the Stem Cell and Tissue Engineering (SCTE) Laboratory for research purposes only. All of the procedures in this study were approved by the Ethics Committee of the Faculty of Medicine Universitas Indonesia (No: KET-689/UN2.F1/ETIK/PPM.00.02/2022). The research was conducted at the SCTE Laboratory and Molecular Biology and Proteomics Core Facilities (MBPCF) of IMERI Faculty of Medicine, Universitas Indonesia.
hUC-MSCs cell culture and treatment: Cryopreserved hUC-MSCs were thawed and expanded in the complete medium consisting of alpha MEM (Gibco, Thermo Fisher Scientific, USA), 10% FBS (HyCloneTM, USA), 1% antibiotic-antimycotic (GipcoM, Thermo Fisher Scientific, United States) and 1% Glutamax (GIBCO, Thermo Fisher Scientific, USA). hUC-MSC were seeded in a 6-well plate at a density of 2×105 cells. The medium was replaced every 3 or 4 days until the cell reached 80-90% confluency. After confluence, the cells were harvested using the enzyme TrypLE Select (Gibco, Thermo Fisher Scientific, USA), and divided for MSCs characterization using flow cytometry or replated for further examination of cell viability, migration, and gene expression. The treatment medium contained basal medium alpha MEM (GibcoMem, Thermo Fisher Scientific, USA), 2% FBS (HyCloneTM, USA), 1% Glutamax (GigcoMix, Thermophysical Fisher, U.S.), and 1% antibiotic-antimycotic (GipcoM, Thermo Fisher Scientific, United States) along with LPS (Santa Cruz Biotechnology, US) at concentrations of 10 or 50 mg/ml. The LPS concentration was determined based on consideration from previous related studies and the optimization before the experiment. Cells were divided into several groups: 1) Cells without intervention (control), 2). Cells treated with 10 mg/ml LPS, and 3) Cells treated with 50 mg/ml LPS. The cells were placed in an incubator with 5% CO2 and 20% O2 at 37°C and harvested at 3 and 6 hr.
MSCs characterization: The cultured cells were harvested after they reached 80-90% confluence, using TripLE SelectTM enzyme (Gibco). Following cell detachment, the complete medium was added to neutralize the enzyme. The cells were then centrifugated at 1200 rpm for 10 min, and the pellet suspended in Phosphate-Buffered Saline (PBS). The cells were washed in PBS by centrifugating at 1200 rpm for 10 min. The pellet was suspended in PBS and prepared for antibody staining for MSCs characterization. BD StemflowTM Human MSC analysis kit was used and the manufacture instructions followed. In brief, the cells were divided into three tubes based on the staining antibody, the isotype, the unstained and the stained tubes. The cells were stained for MSCs markers which include CD90 (FITC Mouse anti-human CD90), CD105 (PerCP-CyTM5.5 Mouse anti-human CD105) and CD73 (APC Mouse anti-human CD73). The cell population was analysed using BD FACSAria III.
Cell viability: Cell viability was measured following the cell counting kit-8 (CCK-8) protocol (Dojindo’s). In brief, hUC-MSCs were seeded in a 96-well plate at a density of 5×103 cells/well in a complete medium, incubated in an incubator with 5% CO2, 20% O2, and a temperature of 37°C until the density reached 80-90%. The complete medium was replaced with a treatment medium and incubated for 3 and 6 hr. The treatment medium was discarded, and the cells were washed with PBS. The cell medium was replaced with a culture medium and 10 ml solution of the CCK-8 solution was added and incubated for 4 hr. The absorbance was read using a microplate reader (Varioskan Lux, Thermo Scientific) at a wavelength of 450 nm. Cell viability was calculated based on the Optical Density (OD) readings obtained from the microplate reader.
Cell migration assay: hUC-MSCs were seeded on a 12-well plate at density of 5×104 cells/well in a complete medium. After 70–80% confluence, the complete medium was replaced with the treatment medium containing LPS (10 and 50 mg/ml) and incubated for 3 and 6 hr. The cells were then scratched using a 100 ml micro-pipette tip along the diameter of the well and incubated for 24 hr. The migrated cell in the scratched area was observed and photographed under an inverted microscope (Nikon Eclipse Ti-S). Cell migration was analyzed using ImageJ® software (software downloaded from https://imagej.nih.gov/ij/) with the wound healing size tool plugin. Cell migration was measured by calculating the percentage of migrating cells.
Quantitative RT-PCR analysis: Quantitative RT-PCR was performed to assess: 1) NLRP-3 inflammasome pathway including IL-1β and Caspase-1, 2) TLR-4 and RelA/p65 expression, and 3) the differentiation capacity of UC-MSCs after LPS induction (using RUNX2, PPARγ and COL 2A1 as markers of differentiation into osteogenic, adipogenic and chondrogenic lineages respectively). The qRT-PCR examination was proceeded by RNA isolation from the cells according to the manufacturer's protocol of the Total RNA Mini Kit (Blood/Cultured Cell) Geneaid. The concentration and purity of RNA were measured using the spectral photometry (Varioskan Lux, Thermo Scientific) machine. The next step was cDNA synthesis using the River TraAce RT Master Mix kit (Toyobo, Japan) following the procedure contained in the kit and using Veriti 96 Well Thermal Cycler Applied Biosystems by Thermo Fisher Scientific. To amplify the cDNA, the primers were used, mixed with SensiFAST SYBR Lo-ROX Kit (Bioline, UK), and analyzed using the 7500 fast Real-Time PCR system (Applied Biosystems). The primer sequences utilized in this study are listed in table 1 14. The data obtained from the qPCR were analyzed using Livak's method (2-Δ ΔCT).
Statistical analysis: The data was analysed using GraphPad Prism for MacOS 10.0.2 software and presented as mean±SEM. Differences between groups were tested using one-way ANOVA and a post-hoc Tukey test. All p<0.05 were considered statistically significant.
Results :
Flow cytometry characterization of UC-MSCs: Surface antigen detection for MSCs showed high levels of the mesenchymal markers CD73 (99.9%), CD90 (100%) and CD105 (98.8%), confirming mesenchymal identity of the cultured UC-MSCs (Figure 1).
The effect of short-term inflammatory environment on hUC-MSCs viability: Short-term exposure of hUC-MSCs to an inflammatory environment with LPS induction showed comparable cell viability compared to that of the control group (Figure 2). LPS induction for 3 hr at both 10 μg/ml (93.28±2.29) and 50 μg/ml (89.03±1.55) showed no significant difference in cell viability from the control group (90.57±1.20). The prolonged induction up to 6 hr showed only a slight decrease in cell viability at 10 μg/ml (89.85±2.37) and 50 μg/ml (89.5±0.91), respectively, compared to the control group (90.88± 1.38). Statistical analysis found no significant difference between the length of exposure and LPS concentration.
The effect of short-term inflammatory environment exposure on hUC-MSC migration: Exposure to an inflammatory environment with LPS induction for 3 and 6 hr had a significant negative effect on the migration percentage of hUC-MSC. Specifically, after 3 hr of LPS induction at 50 mg/ml, hUC-MSC migration was significantly reduced (70.97±8.57, p=0.047) compared to the control group (99.99±0.006). After 6 hr of prolonged LPS induction, both 10 mg/ml (87.06±0.308, p=0.04) and 50 mg/ml (83.89±1.086, p=0.0013) showed a significant decrease in migration compared to the control group (96.16±1.95). Figure 3 demonstrates the significant impact of short-term inflammatory exposure on hUC-MSCs migration, as expressed by the percentage of the closure area.
Effect of short-term inflammatory environment exposure on the expression of NLRP3 inflammasome-related genes in hUC-MSCs: To assess whether short-term inflammatory environment exposure is associated with NLRP3 inflammasome expression in hUC-MSCs, RT-qPCR was performed. The results demonstrated a prominent increase in mRNA levels of the NLRP3 inflammasome (p<0.05) after 3 hr of LPS induction, at both 10 and 50 μg/ml. Prolonged LPS exposure for up to 6 hr showed a significant increase in mRNA levels of NLRP3 inflammasome (7.2±2.63; p=0.024) at the 10 μg/ml concentration compared to the control group, while at 50 μg/ml concentration, the increase was only marginal (Figure 4A).
Increased expression of the NLRP3 inflammasome entails a cascade leading to the upregulation of Caspase-1 and IL-1β. The mRNA levels of Caspase-1 increased with higher LPS concentrations and longer exposure times. Notably, both 10 and 50 μg/ml LPS inductions resulted in an increase of Caspase-1 mRNA levels at 3 hr, with a significant difference observed at 50 μg/ml compared to the control group. Prolonged exposure to LPS for up to 6 hr showed a significant increase in Caspase-1 mRNA levels at both LPS concentrations compared to the control group (Figure 4B). The LPS-induced inflammatory microenvironment also influenced IL-1β expression in hUC-MSCs, with a significant increase in IL-1β mRNA levels observed with higher LPS concentrations and longer exposure times, as illustrated in figure 4C.
Effect of short-term inflammatory environment exposure on the expression of TLR-4 and RelA/p65 in hUC-MSCs: This study examined the upstream pathway through which an inflammatory environment with LPS induction induces NLRP3 inflammasome expression. The LPS-induced inflammation is known through the TLR-4 and NFκB pathway. The RT-qPCR showed a significant increase in the mRNA level of TLR-4 after 3 hr of LPS induction at both 10 μg/ml (2.43±0.42) and 50 μg/ml (2.51±0.39) compared to the control group. Prolonged LPS induction for up to 6 hr showed an increase in the mRNA level of TLR-4, which was significant at 10 μg/ml (1.92±0.24, p<0.001) compared to the control group (Figure 5A).
Examination of RelA/p65 expression, representing of NFκB activation, showed significant increase in mRNA levels following LPS induction compared to the control group. Significant increases in RelA/p65 mRNA levels of (3.61±0.22 and 3.56±0.17) were observed after 3 hr of LPS induction at concentrations of 10 and 50 μg/ml, respectively, compared to the control group. Following a 6-hr LPS induction at both concentrations, the expression of RelA/p65 decreased (2.84±0.16 and 2.64±0.16, respectively), but remained significantly higher than that of the control group, as shown in figure 5B.
The effect of short-term inflammatory environment exposure on hUC-MSC differentiation capacity: To assess whether short-term inflammatory environment exposure affects differentiation capacity of UC-MSCs, the expression of COL 2 A1, RUNX2 and PPARγ which represents for chondrogenic, osteogenic and adipogenic differentiation lineages, were evaluated, respectively. The differentiation capacity into chondrogenic, osteogenic and adipogenic lineages diminishes over time due to prolonged exposure. RT-qPCR revealed a significant decrease in COL 2A1 mRNA levels following 3 hr of LPS induction at a concentration of 50 μg/ml (0.13±0.015; p<0.001). Prolonged exposure of 6 hr showed a significant decrease in COL2AI mRNA levels at both 10 μg/ml (0.10±0.026) and 50 μg/ml (0.25±0.05) compared to control group (Figure 6A). Analysis of RUNX2 expression, indicative of osteocyte differentiation, revealed RUNX2 expression comparable to the control group when stimulated with LPS 10 μg/ml at both 3 and 6 hr. However, a substantial decrease in RUNX2 expression was observed following LPS stimulation at a concentration of 50 μg/ml after 3 hr (0.49±0.046), but it was comparable to the control group at 6 hr of LPS stimulation (Figure 6B).
The expression of PPARγ which represents adipogenic differentiation, was significantly decreased compared to control group, after 3 hr of LPS induction at concentration of 10 μg/ml (0.30±0.091) and 50 μg/ml (0.26±0.03). Prolonged LPS exposure at concentration of 10 μg/ml (0.10±0.013; p<0.001) and 50 μg/ml (0.13±0.012; p<0.001) for 6 hr diminished the mRNA expression levels of PPARγ compared to the control group (Figure 6C).
Discussion :
LPS is commonly used in research to stimulate inflammatory or tissue injury both in vivo and in vitro study 15,16. In this study, LPS was used to create an inflammatory environment that mimics injured tissue where the hUC-MSCs may be applied to promote tissue regeneration. The environment could affect stem cell properties such as viability, migration, and gene expression in response to external stress. However, whether the response of hUC-MSCs to short-term inflammatory environment exposure using LPS induction involves the expression of the NLRP3 inflammasome and its related pathway, remains to be investigated.
Cell viability refers to the physiological status of cells, influenced by microenvironmental elements and responses to stress agents. Maintaining cell viability during transplantation is crucial for tissue regeneration. The present study found no difference in hUC-MSCs viability after LPS induction compared to the control group, suggesting their ability to withstand short-term inflammatory conditions. This aligns with the findings of Kuntjoro Mefina et al 17. However, Hou Sen Yu et al showed a dose-dependent decrease in hUC-MSC viability and increased apoptosis after longer LPS exposure 18. The duration and intensity of inflammation play a significant role in cell survival. This study used shorter exposure times (3 and 6 hr) compared to the longer durations of Hou Sen Yu et al, highlighting the importance of understanding the impact of inflammatory conditions on cell viability 18.
Cell mobility is vital for survival, growth, and response to the environment, playing a key role in various disease processes. Cells exhibit different migratory strategies based on their phenotype and the microenvironment. LPS induction has been shown to impact cell migration in diverse cell types. The present study revealed that short-term LPS induction at 50 μg/ml for 3 and 6 hr reduced the migratory ability of hUC-MSCs. A lower LPS concentration of 10 μg/ml required a longer exposure time (6 hr) to diminish hUC-MSCs migratory ability. This highlights the influence of the inflammatory milieu on hUC-MSC migration. Previous studies have shown that LPS can induce cell migration in different cell types, such as NIH3T3 cells 19, endothelial cells 20, and oligodendrocyte precursor cells 21. LPS may also inhibit the intrinsic mobility of stem cells by triggering internal changes. When LPS binds to cell surface receptors, it initiates an inflammatory response that disrupts the structural elements necessary for cell movement 22. Furthermore, LPS can affect the balance of specific cell membrane proteins, impacting cellular adhesion strength and locomotion 23. Exposure to LPS leads to a reorganization of the cellular structural framework crucial for cell migration 24. LPS exposure can also alter the expression of genes involved in movement and attachment, potentially redirecting cells to areas requiring healing 25.
The biological characteristics of MSCs are influenced by the inflammatory environment they encounter at injured or inflamed sites through Toll-Like Receptors (TLRs). Activation of TLR-4 in MSCs can lead to the translocation of NF-κB into the nucleus, triggering pro-survival signalling pathways and regulating the inflammatory response. This enables MSCs to adapt and survive in stressful and inflammatory conditions 26,27. This study suggests that the TLR4/RelA(p65) pathway plays a role in the biological properties of hUC-MSCs following exposure to LPS. The mRNA levels of TLR4 and ReIA(p65) increased after 3 hr of exposure to LPS at both low (10 μg/ml) and high (50 μg/ml) concentrations, but decreased after 6 hr. This response was accompanied by an increase in the mRNA levels of the NLR3 inflammasome, indicating that the cells effectively detect and respond to LPS through TLR4, leading to the activation of ReIA(p65) and subsequent cellular cascade activities 28. Consistent with previous studies, we observed an upregulation of NLRP3 inflammasome mRNA levels, as well as increased expression of IL-1β and Caspase-1, suggesting a response of hUC-MSCs to the inflammatory environment induced by LPS exposure.
Inflammasome-mediated inflammatory cytokines negatively impact cell properties such as cell junctions 29, migration ability 30, pyroptosis 30, and cellular signaling 30,31. NLRP3 is involved in various biological pathways, and inflammasome-independent NLRP3 activities may have different roles in diverse cell types. The present study demonstrates that the NLRP3 inflammasome is highly expressed in hUC-MSCs under short-term LPS exposure, leading to the expression of IL-1β and Caspase-1, indicating classical activation of NLRP3 inflammasome. Moreover, NLRP3 inflammasome activation was associated with reduced migratory ability in hUC-MSCs, potentially impacts their therapeutic effectiveness. Migration ability is one of MSC's properties involved in tissue repair. MSCs are capable of migrating to the site of lesions in various conditions, principally inflammation, tissue damage, and tumours 32. Understanding the response of MSCs is crucial for their effective clinical application, particularly when dealing with an inflammatory environment.
Pre-treatment of MSCs with a specific TLR ligand could be a useful priming step to adjust one of its functions and achieve the desired therapeutic outcome. Additionally, the presence of inflammation can greatly impact the MSCs differentiation, leading to a deviation from the desired lineages and potentially promoting undesired outcomes like fibrosis. In this study it was found that the differentiation capacity of hUC-MSCs were significantly reduced at the mRNA expression levels (RUNX2, COL 2A1, PPARγ) under LPS stimulation compared to the control group. This finding is in line with other studies which showed deterioration of MSCs differentiation capacity under different inflammatory microenvironment 33-35. Both migration and differentiation capacity are crucial for transplanted MSCs to stimulate tissue regeneration in injured tissue, as shown to be diminished in this study. However, while the recent study highlighted the important role of NLRP3 inflammasome and TLR4/NFκB in the inflammatory response and their impact on MSC behaviours, we have not yet explored other potential pathways that could provide a more nuanced and context-dependent modulation of MSCs in sensing and responding to inflammation, such as TGF-β, AMPK, and mTOR 36,37. Additional research is needed to investigate these alternative pathways.
These findings highlight the significance of exposure duration and the levels of inflammatory environment for hUC-MSCs migratory and differentiation capacity, which in turn affects the successful engraftment of hUC-MSCs. Additionally, the TLR4/NLRP3 inflammasome pathway enables hUC-MSCs to detect inflammatory stress in their local environment.
Conclusion :
In this study, we simulated the inflammatory microenvironment of hUC-MSCs using LPS exposure. Our results highlighted that a short exposure time (3 hr) of LPS has decreased the migration and differentiation in mRNA expression levels of hUC-MSCs, which in part involves the expression of TLR4/RelA(p65) and NLRP3 inflammasome. The findings provide a basis for future studies on the effect of the inflammatory environment on stem cell biology, which is crucial for their successful application in injured tissue.
Acknowledgement :
This study is supported by a research grant from Universitas Indonesia (Hibah PUTI No.NKB-1226/UN2.RST/HKP.05.00/2022). All procedures in this study have been approved by the Ethics Committee of the Faculty of Medicine, Universitas Indonesia (No: KET-689/UN2.F1/ETIK/PPM.00.02/2022).
Conflict of Interest :
The authors declare no competing interests.
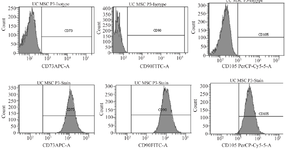
Figure 1. Characterization of cultured UC-MSCs using flow cytometry.
|
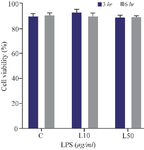
Figure 2. Effect of short-term inflammatory environment exposure with LPS induction on hUC-MSC viability. C = control group, L10 = LPS 10 μg/ml, and L50 = LPS 50 10 μg/ml.
|
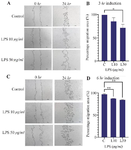
Figure 3. Effect of short-term inflammatory environment exposure with LPS induction on hUC-MSC migration by scratch test. A-B after 3 hr induction; C-D after 6 hr induction (*p=0.0472) (**p< 0.005).
|
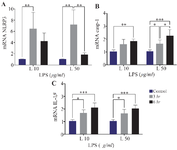
Figure 4. The effect of inflammatory environment exposure with LPS induction on hUC-MSC relative mRNA expression level of NLRP3 inflammasome, Caspase-1, and IL-1β.
*(p=0.024), **(p=0.009), ***(p<0.006).
|
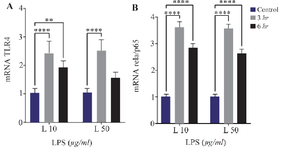
Figure 5. The effect of inflammatory environment exposure with LPS induction, on hUC-MSC relative mRNA expression level of TLR4 and RelA/p65, **(p=0.0098), ****(p<0.0001).
|
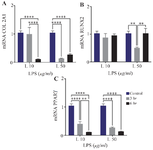
Figure 6. Relative mRNA expression level of COL2AI, PPARγ, and RUNX2 after LPS induction in different concentrations and exposure time. **(p <0,0067), ****(p = <0,0001).
|
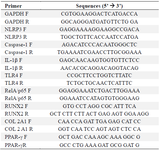
Table 1. Primer sequences
|
|