Nephroprotective and Nitric oxide Scavenging Activity of Tubers of Momordica tuberosa in Rats
-
 Kumar, Pramod
Pramod Kumar, M.Pharm., Department of Pharmacognosy, V. L. College of Pharmacy, Manik Prabhu Temple Road, Raichur-584103, India, E-mail: pramod4407@gmail.com
Kumar, Pramod
Pramod Kumar, M.Pharm., Department of Pharmacognosy, V. L. College of Pharmacy, Manik Prabhu Temple Road, Raichur-584103, India, E-mail: pramod4407@gmail.com
-
Department of Pharmacognosy, V.L. College of Pharmacy, Raichur, India
-
Devala Rao, G.
-
KVSR Siddhartha College of Pharmaceutical Sciences, Vijayawada, India
-
Lakshmayya, -
-
GRD Institute of Management and Technology, Dehradun, India
Abstract: Hydroalcoholic extract (70% ethanol extract) of tubers of Momordica tuberosa Cogn. (Cucurbitaceae) was subjected to preliminary phytochemical screening by qualitative tests. Nitric oxide scavenging activity was performed by Griess reagent method. And nephroprotective activity was assessed in gentamicin, cisplatin and paracetamol induced renal damage in wistar rats (150-200 g) by standard methods. The protective property of 70% ethanol extract was assessed by measuring the levels of body weight, blood urea, serum creatinine, tissue glutathione and lipid peroxidation in administered doses. The extract exhibited free radical scavenging activity in dose dependant manner. And 100 g/ml dose produced significantly higher scavenging activity than standard sodium metabisulphate at 25 g/ml. Also, it significantly reduced the renal damage caused by cisplatin, gentamicin and paracetamol at a dose of 40 mg/kg.
Introduction :
The term renal failure primarily denotes failure of the excretory function of kidney, leading to retention of nitrogenous waste products of metabolism in the blood. In addition, there is failure in regulation of fluid and electrolyte balance along with endocrine dysfunction. The renal failure is fundamentally categorized into acute and chronic renal failure (1,2).
Chronic Renal Failure (CRF) is an irreversible deterioration in the renal function, which classically develops over a period of years, leading to loss of excretory metabolic and endocrine functions. Various causes of renal failure has been attributed to hypertension, diabetes mellitus, antineoplastic agents like cyclophosphamide, vincristin and cisplatin (3).
Acute Renal Failure (ARF) refers to the sudden and usually reversible loss of renal function, which develops over a period of days or weeks. There are many causes of acute renal failure, which could be pre-renal (55%), renal (40%), or post renal (5%). Among the renal causes of acute renal failure, acute tubular necrosis is more common accounting for 85% of incidence. Acute tubular necrosis occurs either due to ischemia or due to toxins. The toxin can be either exogenous or endogenous. The exogenous agents are radiocontrast agents, cyclosporine, antibiotics, chemotherapeutic agents, organic solvents, acetaminophen and illegal abortifacients (1,4).
Ancient literature has prescribed various herbs for the cure of kidney disease (5). The term “Pashanabeda” has been cited in the ancient literature, ayurveda (5) to identify a group of plants, which have been extensively used in the indigenous system of medicine to dissolve urinary calculi and stones.
The plant Momordica tuberosa Cogn (M.tuberosa) (Cucurbitaceae) known as Athalakai in Tamil and Karchikai in Kannada languages, Dravidian languages spoken in India is an important plant traditionally used as abortifacient (6). The plant found growing abundantly in and around Raichur, a district place in the state of Karnataka, India possesses hypoglycemic activity (7). The tubers have abortifacient and antiovulatory activity (8). The fruits contain citric acid and maleic acid (9). Since the fruits of M.tuberosa reportedly contain vitamin C (9), a known antioxidant, it was hypothesized that the tubers may also contain antioxidant principles and hence, selected for phytochemical screening and evaluation of nitric oxide scavenging and nephroprotective activity. We have previously reported the antioxidant and hepatoprotective nature of the extract (10).
Materials and Methods :
Plant material
The tubers of Momordica tuberosa Cogn. were collected from the suburban fields of Raichur, a district place in the state of Karnataka, India during January, 2006 and were identified and authenticated by Prof. Srivatsa, Retired Professor of Botany, L.V.D. College, Raichur. A Herbarium specimen (VLCP-02/ 05) is deposited in the Department of Pharmacognosy, V.L. College of Pharmacy, Raichur.
Preparation of extract
The extraction method consisted of a typical partitioning protocol using immiscible solvents sequentially. The coarse powder of shade-dried tubers of M.tuberosa was extracted sequentially with petroleum ether (40-60°C), chloroform and ethanol (11) by Soxhlet method. Finally, the aqueous extract was prepared by maceration with water for 24 hr (11). Similarly, 70% ethanol extract (TMT) of the tubers was also prepared after defatting the coarse powder. The obtained extracts were dried under reduced pressure by using Rotaflash evaporator.
Preliminary phytochemical screening
All extracts obtained were screened for the presence of different phytochemical groups by using standard chemical tests (11,12).
Animals
Albino rats (150–200 g) of either sex were obtained from Sri Venkateshwara Enterprises, Bangalore and housed in plastic animal cages in groups of 6 animals with 12:12 hr of light: dark cycle under standard husbandry conditions. The animals were fed with standard rodent diet and provided water ad libitum. The animals were used for the study after one week of acclimatization. The approval of Institutional Animal Ethical Committee was obtained prior to the experiments.
Nitric oxide scavenging activity
Nitric oxide scavenging assay was performed using Griess reagent method (13). To 1 ml each of various concentrations of the extract (20-100 g/ml), 0.3 ml of sodium nitroprusside (5 mM) was added. The test tubes were then incubated at 25°C for 150 min. After 150 min, 0.5 ml of Griess reagent (equal volume of 1% sulphanilamide in 5% ortho phosphoric acid and 0.01% napthyl ethylene-diamine in distilled water, used after 12 hr of preparation) was added. The absorbance was measured at 546 nm. The % inhibition of OD was calculated by using the formula:
where OD is Optical density.
Acute toxicity
Acute toxicity of the TMT was determined by using albino mice as per the OECD guidelines 420 (fixed dose method). The LD50 of TMT was found to be 200 mg/kg. Therefore 1/10th (20 mg/kg) and 1/5th (40 mg/kg) doses were selected for further study.
Evaluation of nephroprotective activity in cisplatin induced nephrotoxicity (14)
Four groups of animals were used for the experiment. First group animals were administered saline (1 ml/kg, p.o) for 7 days. Second group animals were administered cisplatin (6 mg/kg, i.v) for 7 days. The animals in third group were administered 70% ethanol extract 20 mg/kg, p.o for 7 days. Animals of fourth group were administered with 70% ethanol extract 40 mg/kg, p.o for 7 days.
On 2nd day, 30 min after respective administration to groups II, III and IV respectively, cisplatin 6 mg/kg, i.v (14) was administered to all animals, once a day on days 1, 4, 5 and 6 and twice a day on days 2 and 3. Kidney tissues and blood samples were collected on the 7th day and assessed for body weight (15), blood urea (16,17) and serum creatinine (18). The animals were sacrificed under mild ether anaesthesia and spinal dislocation.
Evaluation of nephroprotective activity in gentamicin induced nephrotoxicity (19)
Group one, which received only normal saline throughout the course of the experiment was used as control. The second group of animals received daily i.p injection of gentamicin (80 mg/kg) for eight days. This dose has already been shown to produce nephrotoxicity (17). The animals of 3rd group received 80 mg/kg of gentamicin i.p for eight days and 20 mg/kg, p.o of 70% ethanol extract which was started three days prior to the gentamicin injections and continued for eight days with gentamicin. Group four animals were administered 80 mg/kg of gentamicin i.p for eight days and 40 mg/kg of 70% ethanol extract p.o which was started three days prior to the gentamicin injections and continued for eight days with gentamicin. At the end, the animals were subjected to mild ether anaesthesia after blood samples were collected, the kidney tissues dissected out, blotted off blood, washed with saline and stored in 10% formalin and assessed.
Evaluation of nephroprotective activity in paracetamol induced nephrotoxicity (20, 21)
In the dose response experiment, animals were randomly assigned into 4 groups of 6 rats each.
First group animals were administered saline (1 ml/kg, p.o) for 7 days. Second group animals were administered paracetamol 2 g/kg for 7 days. The animals in third group were administered 70% ethanol extract 20 mg/kg p.o for 7 days. Animals of fourth group were administered with 70% ethanol extract 40 mg/kg, p.o for 7 days.
On the 5th day, 30 min after respective administration to Groups I, III and IV paracetamol 2 g/kg was given p.o. After 48 hr of paracetamol administration rats were subjected to mild ether anaesthesia after blood samples collection for evaluating the serum biochemical parameters. Kidney was dissected out, blotted off blood, washed with saline and stored in 10% formalin. The collected blood was centrifuged immediately to get clear serum and subjected to various biochemical studies.
GSH estimation in paracetamol induced nephrotoxicity
Tissue samples were homogenized in ice cold Trichloroacetic acid (1 gm tissue in 10 ml of 10% Trichloroacetic acid) in an ultra turrax tissue homogenizer. Glutathione measurements were performed using a modification of the Ellamn procedure (22). Briefly, after centrifugation at 3000 rpm for 10 min, 0.5 ml supernatant was added to 2 ml of 0.3 M disodium hydrogen phosphate solution. A 0.2 ml solution of dithiobisnitrobenzoate (0.4 mg/ml in 1% sodium citrate) was added and the absorbance at 412 nm was measured immediately after mixing. Percentage of absorbance is directly proportional to the levels of Glutathione. Hence, percentage increase in absorbance was calculated.
Lipid peroxidation in paracetamol induced nephrotoxicity
The degree of lipid peroxide formation was assessed by monitoring thiobarbituric reactive substance formation (22,23). Stock solution of TCA-TBA-HCl reagent (15 %w/v trichloroacetic acid; 0.375 % w/v thiobarbituric acid; 0.25N hydrochloric acid) was prepared. Combined 1.0 ml of biological sample (0.1-2.0 mg of membrane protein or 0.1-0.2 mol of lipid phosphate) with 2.0 ml of TCA-TBA-HCl reagent and mixed thoroughly. The solution was heated for 15 min in a boiling water bath. After cooling, the flocculent precipitate was removed by centrifugation at 1000 rpm for 10 min. The absorbance of the sample was measured at 535 nm against a blank that contained all the reagents except the lipid. The malondialdehyde concentration of the sample was calculated by using an extinction coefficient of 1.56 105 M–1 cm–1.
Statistical analysis
Results were expressed as mean SEM. Statistical analysis was performed with one way analysis of variance (ANOVA). P value less than 0.05 was considered to be statistically significant.
Result :
The preliminary phytochemical investigations showed the presence of sterols in the petroleum ether extract, saponins, cardiac glycosides, triterpenoids and bitters in the ethanol extract, and carbohydrates and constituents of ethanol extract in water extract. The phytoconstituents present in the 70% ethanol extract were similar to that of ethanol and aqueous extracts. All tests were performed by comparison of the results with standard in each group. The presence of saponins was confirmed by the persistent foam test and haemolysis test (11,12).
The nitric oxide scavenging activity of 70% ethanol extract was studied (Table 1). The extract showed dose dependent inhibition compared to standard drug, sodium metabisulphite. The extract at a dose of 100 g/ml had significantly higher scavenging activity than standard at 25 g/ml.
Administration of cisplatin caused decrease in body weight upto 14.07 % (Table 2). The treatment with TMT restored body weights in dose dependent manner with 40 mg/kg dose restoring it to 6.57%. Blood urea nitrogen level which increased with cisplatin treatment to 83.78 mg/dl, was restored to 35.32 mg/dl with 40 mg/kg dose of the extract. Serum creatinine level which, increased to 2.53 mg/dl was restored to 0.85 mg/dl with 40 mg/kg dose.
The gentamicin administration caused reduction in body weight up to 10.31% and increased blood urea to 59.68 mg/dl and creatinine to 1.74 mg/dl (Table 3). The extract restored the weight reduction to 6% and blood urea to 30.23 mg/dl at 40 mg/kg dose. The creatinine was significantly reduced to 1.12 mg/dl at the same dose.
Paracetamol administration at a dose equal to 2 g/kg dose revealed an increase in blood urea and serum creatinine levels (56.39 and 1.96 mg/dl) as compared with control (29.28 and 0.68 mg/dl). The extract restored their levels significantly to near normal at 33.68 and 0.79 mg/dl respectively at a dose equivalent to 40 mg/kg (Table 4). It also reduced lipid peroxidation and prevented the depletion of tissue glutathione (GSH) levels (Tables 5 and 6).
The free radicals play very important role in human health and beneficial in combating against several diseases. Reactive Oxygen Species (ROS) is generated during various metabolic activities. Contaminants in the environment as well as normal metabolism of a cell can change molecule into a free radical. The examples of ROS are OH, O2, H2O2, O3, HOCI, RO2 and RO. The plants are susceptible to damage caused by active oxygen and thus develop numerous antioxidant defense systems resulting in formation of numerous potent antioxidants.
Many aromatic, medicinal and spice plants contain compounds that possess confirmed strong antioxidative components. Some potent antioxidants are chlorophyll derivatives, essential oils, carotenoids, alkaloids, phytosterols, phenolics- coumarines, flavonoids, polyphenolics, tannins etc. Usually, polyphenols and carotenoid pigments, being the major nutritional antioxidants in food, attract most of the research in this area. Some saponins have also been found to have antioxidative or reductive activity. A group of saponins produced in legumes, namely, group B soya saponins, contain an antioxidant moiety attached at C23 (24). This unique sugar residue, 2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP), allows saponins to scavenge superoxides by forming hydro peroxide intermediates, thus preventing bio-molecular damage by free radicals.
Discussion :
While plant extracts containing saponins have been widely used in food and other industrial applications mainly as surface active and foaming agents (25) saponins in foods have traditionally been considered as "antinutritional factors" (26) and in some cases have limited their use due to their bitter taste (27). Therefore, most of the earlier research on processing of saponins targeted their removal to facilitate human consumption. However, food and non-food sources of saponins have come into renewed focus in recent years due to increasing evidence of their health benefits such as cholesterol lowering and anticancer properties (28). Recent research established saponins as the active components in many herbal medicines and highlighted their contributions to the health benefits of foods such as soybeans (29,30) and garlic (31).
Conclusion :
Since, 70% ethanol extract of tubers of M.tuberosa contain saponins and triterpenoids, the antioxidant and organ protective properties of it could be attributed to the presence of these constituents. To conclude, our studies have shown that the tubers of M.tuberosa possesses marked nephroprotective activity and could have promising role in the treatment of acute renal injury induced by nephrotoxins. This activity may be attributed to its antioxidant property. Further work is on in our laboratory to isolate and characterize phytoconstituents responsible for nephroprotective property.
Acknowledgement :
Authors wish to express their gratitude to Managements of V.L. College of Pharmacy, Raichur, India and SCS college of Pharmacy, Harapanahalli, India for providing necessary facilities in conducting this research work.
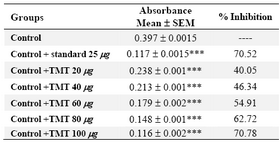
Table 1. In vitro nitric oxide scavenging activity of 70% ethanol extract of tubers of M.tuberosa
(Values expressed as absorbance are the mean ± SEM, n= 3, Significance *** p<0.001 compared to control, Std: Sodium metabisulphate)
|
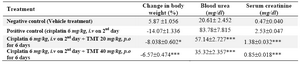
Table 2. Effect of 70% ethanol extract of M.tuberosa tubers in cisplatin induced renal damage in rats
(Values are mean ± SEM, n=6, Significance *p<0.05, **p < 0.01 and ***p<0.001 compared to control)
|
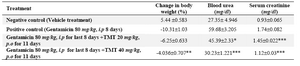
Table 3. Effect of 70% ethanol extract of M.tuberosa tubers in gentamicin induced renal damage in rats
(Values are mean ± SEM, n=6, Significance *p<0.05, **p < 0.01 and ***p<0.001 compared to control)
|
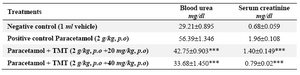
Table 4. Effect of 70% ethanol extract of M.tuberosa tubers in paracetamol induced renal damage in rats
(Values are the mean ± SEM, n=6. Significance ***p<0.001 compared to control)
|
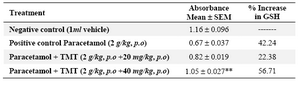
Table 5. Effect of 70% ethanol extract of M.tuberosa tubers on tissue GSH level in paracetamol induced nephrotoxicity
(Values are the mean ± SEM, n=6. Significance *p<0.05 and **p<0.01compared to paracetamol treatment, TMT- 70 % ethanol extract)
|
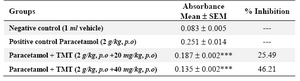
Table 6. Effect of 70% ethanol extract of M.tuberosa tubers on tissue lipid peroxidation level in paracetamol induced nephrotoxicity
(Values expressed as absorbance are the mean ± SEM, n= 6. Significance ***p<0.001, compared to paracetamol treatment)
|
|