Investigation of Anti-Cancerous Effects of L. casei –ATCC-393 and L. rhamnosus-GG on Apoptosis and Cell Cycle of B- CPAP Thyroid Cancer Cell line in Comparison to Fibroblast Cell Line
-
Honardoost, Maryam
-
Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences, Tehran, Iran
-
Cardio-Oncology Research Center, Rajaie Cardiovascular Research Institute, Iran University of Medical Sciences, Tehran, Iran
-
 Soleimanifar, Fatemeh
Department of Medical Biotechnology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran, Tel: +98 26 34287339; Fax: +98 26 32558931; E-mail: maryamsoleimani03@gmail.com
Soleimanifar, Fatemeh
Department of Medical Biotechnology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran, Tel: +98 26 34287339; Fax: +98 26 32558931; E-mail: maryamsoleimani03@gmail.com
-
Eslami , Solat
-
Department of Medical Biotechnology, School of Medicine, Alborz University of Medical Sciences, Karaj, Iran
-
Cheraghi, Sara
-
Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences, Tehran, Iran
-
Khamseh, Mohammad Ebrahim
-
Endocrine Research Center, Institute of Endocrinology and Metabolism, Iran University of Medical Sciences, Tehran, Iran
-
Darvish, Maryam
-
Department of Medical Biotechnology, Faculty of Medicine, Arak University of Medical Science, Arak, Iran
-
Haddad Kashani , Hamed
-
Anatomical Sciences Research Center, Institute for Basic Sciences, Kashan University of Medical Sciences, Kashan, Iran
Abstract: Background: Thyroid cancer is the most common type of cancer affecting the endocrine system. The main treatment approaches consist of surgical procedures and radioiodine therapy. Recently, there has been a heightened interest in investigating alternative treatment options, including probiotics, which could potentially minimize toxicity. Consequently, there is a growing imperative for research aimed at investigating the potential role of probiotics in the management of cancer.
Methods: The B-CPAP cell line was maintained in culture and tested with different dilutions of two bacterial strains. Toxicity evaluations were performed using the MTT assay to identify appropriate concentrations. mRNA was extracted and analyzed via real-time PCR to measure the expression levels of the Bcl-2, Bax, and P53 genes. Furthermore, changes in the cell cycle and the induction of apoptosis were examined using flow cytometry.
Results: The supernatant derived from Lacticaseibacillus casei (L. casei) – ATCC-393 and Lacticaseibacillus rhamnosus (L. rhamnosus)-GG demonstrated a significant inhibitory effect on the growth of B-CPAP cancer cells. The findings indicated that the combination produced a more pronounced anti-cancer effect by enhancing the expression of pro-apoptotic genes while reducing Bcl-2 gene expression in B-CPAP cells.
Conclusion: The findings indicated a notable change in the expression of genes associated with apoptosis and modifications in the cell cycle. This implies that probiotics may enhance the efficacy of chemotherapy in treating thyroid cancer. In particular, L. rhamnosus and L. casei may play a beneficial role in the therapeutic process. Further research is required to investigate the direct impact of probiotics on thyroid function.
Introduction :
Papillary Thyroid Carcinoma (PTC) represents the most prevalent form of thyroid cancer, with an estimated occurrence of 80% in developed nations 1. The reported 5-year survival rate for this malignancy exceeds 95%; however, a minority of patients (3-5%) experience disease recurrence, which is indicative of a less favorable prognosis 2. Research indicates that a disrupted gut microbiome correlates with an increased risk of several cancers, largely attributable to its influence on the immune system. On the other hand, modifying the gut microbiome has been found to bolster the body's anticancer defenses 1. Studies have shown that the levels of thyroid hormones can have an impact on the gut microbiome, and conversely, the composition of the gut microbiome can influence the activity of endocrine glands, such as the thyroid, through the brain-gut axis 3. Moreover, the gut microbiome is crucial for thyroid function, as it affects nutrient absorption, thereby impacting the endocrine glands and playing a role in the regulation of thyroid hormones and immune responses 4. Some studies suggest that an imbalanced gut microbiome composition correlates with thyroid hormone levels, the development of autoimmune thyroid disorders (such as Graves' disease and Hashimoto's thyroiditis), and the incidence of thyroid cancer 1,2.
Probiotics, including Lactobacilli, are beneficial non-pathogenic microorganisms found in certain food products. When ingested in appropriate quantities, these probiotics can positively influence human health. They are commonly employed in the management of various gastrointestinal conditions, such as diarrhea, and play a role in mitigating infection and inflammation. Additionally, probiotics have the capacity to modulate and maintain the balance of the intestinal microbiome 5,6. Micro-organisms possess the ability to influence the immune system positively, enhancing its function while also exhibiting anti-cancer properties. They achieve this by detoxifying carcinogenic substances and augmenting antioxidant activity. Furthermore, these micro-organisms may contribute to the eradication of cancer cells through the suppression of cellular proliferation and the promotion of apoptosis 6,7. Investigations have demonstrated that probiotics enhance the expression of Bax, which in turn triggers the activation of caspase 3 and caspase 9, along with the release of cytochrome C, culminating in the induction of apoptosis. In addition, these probiotics are associated with a reduction in the expression of genes that facilitate cell cycle progression and the anti-apoptotic gene Bcl-2 6,8.
Various investigations have revealed that lactobacilli can exert inhibitory effects on colon cancer by lowering the activity of tumor-promoting enzymes, binding to mutagenic agents, increasing levels of short-chain fatty acids, reducing pH, and enhancing immune responses. These actions suggest a potential therapeutic role for Lactobacilli in colon cancer management. Current cancer therapies frequently harm healthy cells, leading to toxicity and side effects for patients. Furthermore, the challenge of chemotherapy resistance has become a significant concern. As a result, there is an urgent need to identify novel treatment methods and therapeutic agents, including the exploration of probiotics for their anticancer potential 8,9. Contemporary cancer therapies are increasingly emphasizing the use of agents that demonstrate enhanced efficacy while minimizing toxicity. Probiotics have emerged as a noteworthy consideration in this context, as they are widely regarded as safe owing to their extensive history of human use. Consequently, there is a growing imperative for further investigation into the role of probiotics in cancer treatment 10. Research investigating the impact of probiotic intake on cancer appears to be encouraging, as both in vitro and in vivo studies have recently indicated that probiotic bacteria may lower the risk, occurrence, and quantity of tumors in the colon, liver, and bladder 8,10. Current evidence indicates that probiotics have a beneficial impact on the prevention and management of colon cancer 10. The objective of this research was to explore the potential impacts of Lacticaseibacillus casei (L. casei)–ATCC-393 and Lacticaseibacillus rhamnosus (L. rhamnosus)-GG as promising biological agents, specifically focusing on their anti-proliferative, apoptotic, and cell cycle arrest characteristics in the context of papillary thyroid cancer.
Materials and Methods :
Preparation of bacterial cell-free supernatant: The micro-organisms utilized in this study, namely L. casei –ATCC-393 and L. rhamnosus-GG, were obtained from the Iranian Biological Resource Center. Both bacterial strains were cultured in Man–Rogosa–Sharpe (MRS) broth and subjected to incubation for 24 hr at a controlled temperature of 37°C. After cultivation, the resulting colonies were further inoculated into a fresh liquid MRS medium and incubated for an additional 24 hr. The bacterial culture was subsequently sub-cultured in new MRS medium, and its absorbance was calibrated to a range of 600-650 nm (equivalent to 0.5 McFarland standard), representing approximately 1×107 cfu/ml. Following this procedure, the bacterial culture was centrifuged, and the supernatant was sterilized through a 0.22 μm syringe filter. Subsequently, different dilutions of the supernatant were created using MRS broth.
Co-culture cells with bacteria: The B-CPAP cell lines, derived from PTC, along with fibroblast cells representing a normal cell line, were acquired from the Stem Cell Technology Resource Center and Pasteur Institute of Iran. These cell types were cultured in six-well plates, with transwells positioned in each well. Subsequently, 500 μl of L. casei was introduced into the upper section of one well, L. rhamnosus into another, and a combination of both bacteria into the third well. Following this, the cells were exposed to varying concentrations of probiotic bacterial supernatant (25, 50, 75, and 100 mg/ml) for a duration of three hr.
Cell viability assay: In summary, a total of 1.2×104 cells/well were plated into 96-well plates and incubated overnight in a CO2 incubator at 37°C for a duration of 24 hr. Following this incubation period, the cells were exposed to varying concentrations of probiotic bacterial supernatant (25, 50, 75, and 100 mg/ml) for a period of 3 hr. After treatment, the culture medium was discarded, and the cells were rinsed twice with Phosphate-Buffered Saline (PBS). To assess the cytotoxic effects of the probiotic bacterial supernatant on the cells and to conduct a proliferation assay, the MTT [3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide] colorimetric assay was employed. Subsequently, 100 μl of MTT was introduced into each well, and the reaction was maintained at 37°C in a 5% CO2 atmosphere for 4 hr. The MTT solution was then removed, and the formazan crystals were solubilized in 100 μl of Dimethyl Sulfoxide (DMSO). The absorbance was finally measured at 560 nm using an ELISA reader to determine cell viability.
Fluorescent staining: After a 3-hr period of cell culture and treatment, each well received 500 μl of 0.05% trypsin, which was incubated at 37°C for 10 min to enable the detachment of cells from the culture dishes. Following this step, the cells were washed twice with PBS and subsequently collected by centrifugation at 1500 rpm for 5 min. To evaluate the apoptotic cells, dual fluorescent staining solutions consisting of 1 μl of 100 μg/ml Acridine Orange (AO) and 100 μg/ml Propidium Iodide (PI) were added to each suspension. The morphology of the apoptotic cells was then examined using a fluorescent microscope.
Detection of apoptosis by Annexin V-FITC: The DNA from the isolated cells is stained utilizing the Fluorescein-Isothiocyanate (FITC) annexin V apoptosis detection kit, and flow cytometric analysis is performed within one hour of staining. For each treatment group, 10,000 cells are counted, enabling the quantification of apoptotic and total cell death. The ratios of apoptotic to necrotic cells are then evaluated.
Cell cycle analysis: Cells are stained with the cell cycle kit and subsequently incubated on ice for 10 min. The cell cycle profile is evaluated through the use of a flow cytometer. The data collected are analyzed with Flow Jo software and expressed as the percentage of the cell cycle. Information pertaining to the Real-time PCR reaction and the thermal program has been documented in earlier research.
Genes expression evaluation: Total RNA was extracted from serum using a Trizol-based protocol. In this procedure, 1.5 ml of serum was combined with 0.4 ml of Trizol reagent (Invitrogen, California, USA) and allowed to incubate at room temperature for 5 min. Following this, 80 ml of chloroform was introduced, and the samples were mixed by inversion for 5 min. After a 3-min incubation, the samples were centrifuged at 12,000×g for 15 min at 4°C. The upper aqueous phase was then carefully transferred to a new tube, to which 500 µl of isopropanol was added, followed by a 10-min incubation at room temperature. The resulting pellets were washed with 1 ml of 75% ethanol, vortexed, and subsequently centrifuged at 7,500×g for 5 min at 4°C. The sediment was collected and re-suspended in 50 μl of RNase-free water. The concentration and purity of the total RNA were evaluated using a NanoDrop Spectrophotometer, measuring the Optical Density (OD) ratios of A260/280 nm and A260/230 nm. cDNA synthesis from the extracted total RNA was conducted using the PrimeScript™ RT reagent kit (Takara Company), following the manufacturer's guidelines. The quantitative real-time PCR was performed with the Master Mix q real-time PCR kit from Bayerpol Iran (catalog number: BP-161), adhering to the manufacturer's instructions, utilizing the ABI Prism 7000 HT sequence detection system (Applied Biosystems, America) along with gene-specific primers for p53, Bax, and Bcl2, and GAPDH as the internal control.
Statistical analysis: All assays were conducted in triplicate, and the results were expressed as mean±standard deviation for the measurements. The significance of the differences (p<0.05) was assessed using one-way ANOVA, employing the Statistical Package for the Social Sciences (SPSS) software (Version 26).
Results :
Cell viability assay: The MTT assay for cell viability analysis indicated that probiotics have a cytotoxic impact on cancer cells. B-CPAP cells were treated with the bacterial supernatant and subsequently incubated with MTT solution. As depicted in figure 1, the decrease in cancer cell viability was progressively enhanced by the probiotics L. rhamnosus -GG and L. casei–ATCC-393 in accordance with the dosage administered (Table 1 and Figure 1). The cytotoxic effects of various bacterial concentrations were evaluated individually on the B-CPAP cell line, alongside a control of fibroblast cells (Table 2). As shown in figure 1, there was a clear dose-dependent decline in the viability of B-CPAP cells. Particularly, the probiotics L. rhamnosus-GG and L. casei–ATCC-393 were associated with a significant reduction in B-CPAP cell viability (Figure 1). At a concentration of 100 mg/mL, L. rhamnosus GG demonstrates a reduction in cell viability of up to 82.98%, whereas L. casei ATCC 393 results in a viability decrease of up to 69%. The synergistic effect of these two probiotics leads to a reduction in cancer cell survival by as much as 70%. Notably, the presence of these bacteria does not have a significant impact on the survival rates of fibroblast cells.
The T-test results indicate that L. casei–ATCC-393 at a concentration of 50 mg/ml and L. rhamnosus-GG at 75 mg/ml led to a reduction in the viability of cancer cell lines when compared to normal fibroblast cells. Furthermore, the combination of L. casei –ATCC-393 and L. rhamnosus-GG at concentrations of 75 and 100 mg/ml exhibits the most pronounced anti-cancer effects. However, statistical analysis reveals that none of the probiotic concentrations result in a statistically significant reduction in the survival of cancer cells relative to fibroblast cells.
Flow cytometry: The assessment of early apoptosis was conducted by measuring the protein expression of Annexin V, which serves as an early indicator of apoptosis. Flow cytometry findings demonstrated a considerable increase in apoptosis and necrosis in B-CPAP cells treated with L. casei–ATCC-393 or L. rhamnosus-GG strain GG, compared to the control B-CPAP cell line. Therefore, the use of these probiotics is associated with a reduction in the viability of tumor cells.
Results of induction of apoptosis and necrosis in B-CPAP cell line treated with L. rhamnosus -GG (R) and L. casei–ATCC-393 (C) and combination of two bacterial strains (C+R).
In the control group, nearly all B-CPAP cells (99.1%) were viable. A minimal fraction of the cells was in the delayed apoptosis phase (0.863%), and an even smaller portion was in the early apoptosis phase (0.011%). The percentage of necrotic cells was also very low (0.011%). In contrast, when cancer cells were treated with L. casei–ATCC-393 (denoted as C), there was a significant shift in cell viability. Only 27.6% of the cells survived, while 63.1% entered the early apoptosis phase and 9.17% progressed to late apoptosis. The necrotic cell percentage remained very low at 0.043%. Conversely, exposure to L. rhamnosus -GG (indicated by R) resulted in a higher survival rate, with 57.8% of the cells remaining alive.
However, a significant proportion of the cells were found to be in the early stages of apoptosis, accounting for 64.8%, while 13% were identified as being in the late stages of apoptosis. The necrotic cell population was minimal, at just 0.038%. Following treatment with a combination of the two bacteria, only 30% of the cancer cells survived. In this scenario, 52.7% of the cells were in early apoptosis, and 15.3% were in late apoptosis, with necrotic cells slightly higher at 0.203%. These findings indicate that the dual bacterial treatment exhibits a more potent anti-cancer effect. Observations of cell rupture and fragmentation were noted in the co-culture involving both bacteria. Fluorescent staining results confirmed the occurrence of apoptosis after treatment with L. rhamnosus -GG (R) and L. casei–ATCC-393 (C) (Table 2, Figure 2).
Results of induction of apoptosis and necrosis in fibroblast cell line treated with L. rhamnosus -GG (R) and L. casei –ATCC-393 (C) and combination of two bacterial strains (C+R).
The results of the study reveal that the control group exhibited a high viability of fibroblast cells, with 99.8% remaining alive. A minimal proportion of cells were observed in the delayed apoptosis (0.032%), early apoptosis (0.038%), and necrotic (0.145%) phases. In contrast, the group treated with L. casei-ATCC-393 (C) demonstrated a significant decline in cell viability, with only 36.1% of cells alive. This group showed 42.8% of cells in the delayed apoptosis phase, 20.4% in early apoptosis, and 0.678% in necrosis. Similarly, fibroblast cells subjected to L. rhamnosus -GG (R) treatment displayed a viability of 29.5%, with 33.1% in early apoptosis, 35.9% in delayed apoptosis, and a necrotic percentage of 1.48%. Finally, the combination treatment of both bacterial strains resulted in 25.7% of cells remaining alive, with 36% in early apoptosis, 26.3% in delayed apoptosis, and 11.9% exhibiting necrosis (Table 3, Figure 3).
Gene expression results: We conducted a comparison of gene expression profiles between B-CPAP and fibroblast cells using real-time PCR. The results indicated that P53 and Bax were overexpressed across all B-CPAP bacterial co-culture groups in relation to the control (Table 4). Notably, while Bcl-2 expression did not show significant differences in L. rhamnosus (B-CPAP) or L. casei (B-CPAP), it exhibited a meaningful decrease in both L. rhamnosus and L. casei (B-CPAP) when compared to the control. Furthermore, P53 levels were significantly lower in the fibroblast + R+C group compared to the normal group, and also lower in B-CPAP with or without L. rhamnosus in comparison to the fibroblast cell line. An increase in P53 levels was noted in L. casei (B-CPAP) and L. rhamnosus, L. casei (B-CPAP) relative to the fibroblast group. A decrease in Bcl-2 levels was observed in L. rhamnosus and L. casei (B-CPAP) as well as in L. rhamnosus fibroblast when compared to the control, and in L. rhamnosus and L. casei (B-CPAP) when compared to the fibroblast. Conversely, an increase in Bcl-2 levels was found in B-CPAP, L. rhamnosus (B-CPAP), and L. casei (B-CPAP) compared to the fibroblast. Interestingly, Bcl-2 levels decreased in L. rhamnosus and L. casei (B-CPAP) when compared to the fibroblast.
Discussion :
The implications of utilizing L. casei ATCC-393 and L. rhamnosus GG (LGG) in cancer therapy are significant, as they represent a novel approach to enhancing patient outcomes. By protecting non-cancerous cells and inducing cell death in tumors, these probiotics may play a crucial role in developing more effective and less harmful cancer treatment strategies.
L. casei ATCC-393 and L. rhamnosus GG (LGG) are recognized for their potential anti-cancer properties in oncology. These probiotics can protect healthy human cells while facilitating the death of cancer cells. Available evidence indicates that the health condition of human target cells may play a significant role, as non-transformed cells appear to benefit from LGG, whereas cancer cells seem to experience adverse effects from it. Various molecules derived from LGG, such as p40, p75, HM0539, and bacteriocins, have been recognized for their anti-apoptotic properties towards non-cancerous cells. Additionally, LGG is capable of secreting substantial amounts of short-chain fatty acids, including acetate, propionate, and butyrate, which contribute positively to the preservation of intestinal homeostasis in the host. Additionally, in cases of non-thyroid tumors, L. casei ATCC-393 and L. rhamnosus GG have been found to stimulate a significant anti-tumor immune response. Conversely, the proteins Bax, Bcl-2, and P53 are essential in the mechanisms of cancer development and the efficacy of anti-cancer therapies, with Bax promoting apoptosis, Bcl-2 inhibiting it, and P53 serving as a crucial tumor suppressor. Consequently, upregulation of Bax and P53, alongside the downregulation of the Bcl-2 gene, presents a potential anti-cancer approach. Our gene expression analysis comparing B-CPAP cell lines to fibroblasts revealed that the Bcl-2 gene was significantly expressed, whereas the P53 gene exhibited expression levels below 50% in the untreated PTC cancer cell line relative to the untreated fibroblast group (Figure 4). Notably, the gene expression profiles altered in cell lines treated with bacteria (Figure 5). Specifically, in the groups treated with L. casei -ATCC-393 and L. rhamnosus-GG, there was a marked decrease in Bcl-2 expression, while levels of Bax and P53 expression were found to be elevated compared to the untreated cell lines. The flow cytometry results revealed that in both treated groups, only one-third of the cells remained viable, while the others were either in the early or delayed stages of apoptosis or in a necrotic state. Furthermore, cells exposed to a combination of both bacterial strains exhibited a lower viability and a higher incidence of apoptotic or necrotic conditions. This suggests that the liquid component of the strain contains agents that may inhibit cell proliferation. Previous studies have also highlighted the capacity of Lactobacillus to impede the proliferation of cancer cells. For example, the research conducted by Choi et al in 2005 showed that extracts of Lactobacillus acidophilus (L. acidophilus) resulted in reduced cell survival when compared to the control group. Likewise, the 2015 study by Soltan Dallal et al investigated the anticancer effects of the liquid portion of L. acidophilus on Caco-2 cells, employing a concentration similar to that used in this project 11. Kahouli and colleagues performed an assessment of the cytotoxic effects of Lactobacillus fermentum supernatant on colorectal cancer cells 12. Their results were nearly the same as those obtained in this study. Conversely, the study conducted by Er S et al, which investigated the anti-cancer properties of various lactobacilli on Caco-2 cells, failed to yield significant findings 13.
Probiotics may play a significant role in decreasing the occurrence of complications in individuals diagnosed with thyroid cancer by modifying the composition of both oral and gut microbiota. A supplementation strategy that includes Bifidobacterium infantis (B. infantis), L. acidophilus, Enterococcus faecalis (E. faecalis), and Bacillus cereus (B. cereus) has demonstrated efficacy in alleviating complications associated with thyroid cancer and in reestablishing the equilibrium of the oral and gut microbiota 14. Evidence suggests that probiotic intake may alter the metabolites of gut microbiota, facilitating interactions with neurotransmitters through the gut-thyroid axis, which enhances the functioning of the thyroid gland 15. Furthermore, incorporating prebiotics into the diet serves as an additional approach to enhance gut microbiota, which may result in better thyroid function and a decreased likelihood of developing thyroid cancer 16.
The findings indicate that the combination of two probiotics significantly influences expression changes, thereby positively affecting the apoptosis process in an in vitro setting. These results imply that L. casei– ATCC-393 and L. rhamnosus-GG, as probiotics, can synergistically enhance the efficacy of the anti-cancer drug. Advancements in the comprehension of microbiome alterations in patients with thyroid cancer may lead to the identification of several targeted therapies that exhibit significant clinical efficacy in the future.
Conclusion :
This research explored the influence of the probiotic strains L. casei–ATCC-393 and L. rhamnosus–GG on B-CPAP thyroid cancer cells. The outcomes revealed that both strains, when applied individually or collectively, are capable of initiating apoptosis and affecting the cell cycle in this specific cancer cell line. Therefore, these probiotics might be considered as an adjunctive option in cancer treatment strategies. The implications of the results indicate that the inclusion of lactobacilli probiotics in dietary practices could potentially decrease the chances of developing thyroid cancer.
Conflict of Interest :
Authors declare no conflict of interest.
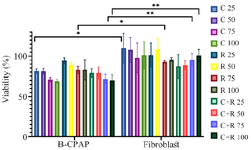
Figure 1. Effect of lactobacilli supernatant on B-CPAP cell viability (%). MTT-based viability assay of fibroblast and B-CPAP culture after 3 hr in contact with a spectrum of different concentrations of L. rhamnosus-GG (R) and L. casei–ATCC-393 (C) and a combination of two bacterial strains (C+R). The t-test was used to calculate statistical significance with p≤0.05 being considered significant.
|

Figure 2. A) B-CPAP (Control), B) B-CPAP treatment with L. casei –ATCC-393, C) B-CPAP treatment with L. rhamnosus-GG. D) fibroblast with L. casei–ATCC-393+L. rhamnosus-GG. Flow cytometric examination of apoptosis and cell cycle. a Flow cytometric examination of apoptosis, necrosis and cell viability–the Annexin V-FITC assay of B-CPAP cells after treatment with L. casei–ATCC-393 (C) and L. rhamnosus-GG (R). Four quadrants of plots depict the following: lower left – viable cells (Annexin V–, PI–), upper left–early apoptotic cells (Annexin V+, PI–), upper right–late apoptotic cells (Annexin V+, PI+), lower right – necrotic cells (Annexin V–, PI+).
|
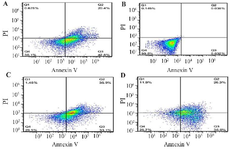
Figure 3. A) fibroblast (Control), B) fibroblast treatment with L. casei–ATCC-393 (C), C) fibroblast treatment with L. rhamnosus-GG (R). D) fibroblast with L. casei–ATCC-393 (C)+L. rhamnosus-GG (R). Flow cytometric examination of apoptosis and cell cycle. Flow cytometry examination of apoptosis, necrosis and cell viability–the Annexin V-FITC assay of fibroblast cells after treatment with L. casei–ATCC-393 (C) and L. rhamnosus-GG (R). Four quadrants of plots depict the following: lower left–viable cells (Annexin V–, PI–), lower right–early apoptotic cells (Annexin V+, PI–), upper right –late apoptotic cells (Annexin V+, PI+), upper left–necrotic cells (Annexin V–, PI+).
|

Figure 4. Analysis of Bcl-2, Bax and P53 gene expression in B-CPAP cell lines compared to fibroblasts. The Bcl-2 genes expressed near to 16-fold changes more than in fibroblast cells, the P53 gene expressed less than 50% in fibroblast group. As for the Bax gene, its expression was nearly the same in both B-CPAP and fibroblast group (p<0.05).
|
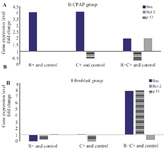
Figure 5. A) Analysis of genes expression in B-CPAP cell and fibroblast lines treated with L. casei–ATCC-393 (C) and L. rhamnosus-GG (R) compared to the control group in RT-PCR reaction. 7A) Bax gene expression increased in all treatment group. In the group of B-CPAP cells treated with R+C+, a significant reduction in the expression of Bcl-2 gene and up regulation of p53 gene expression were observed. Bcl-2 mRNA has decreased in C+B-CPAP group (p<0.05). Untreated B-CPAP cell line was used as control. Bax: pro-apoptotic protein. Bcl-2: anti apoptotic protein. P53: Tumor suppressor protein. B): Bax gene expression increased in treated C+ and C+R+fibroblast cell groups. P53 expression was significantly lower in R+C+ Fibroblast compared with control. While, an increase in the expression of the Bax and Bcl-2 genes was seen in the same fibroblast group. Bcl-2 was downregulated in R+fibroblast cells (p<0.05). Untreated fibroblast cell line was used as control.
|
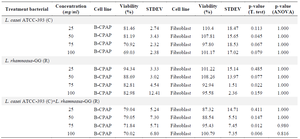
Table 1. Effect of lactobacilli supernatant on B-CPAP and fibroblast cells viability
Concentration-dependent cytotoxic effects of probiotics L. rhamnosus-GG (R) and L. casei –ATCC-393 (C) on B-CPAP (cancer cell line) and comparison with fibroblasts. The table reported the maximum effect (% viability) calculated per each cell type tested. N = 3. Values are presented as Mean ± SD.
|
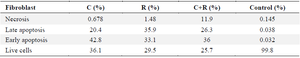
Table 2. Results of induction of apoptosis and necrosis in fibroblast by bacteria
|
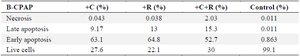
Table 3. Results of induction of apoptosis and necrosis in B-CPAP cell line by bacteria
|
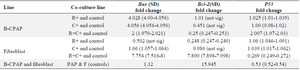
Table 4. Gene expression profiles in B-CPAP and fibroblast Co-culture line
|
|