Revolutionary Regeneration Therapy Utilizing Dental Stem Cells and State-of-the-Art Nanotechnology Devices to Heal Injured Teeth and Tissues
-
 Balfaki, Mohammad
Shahid Beheshti University, Tehran, Iran, Tel: +98 9359430494; E-mail: m.balfaki@alumni.sbu.ac.ir
Balfaki, Mohammad
Shahid Beheshti University, Tehran, Iran, Tel: +98 9359430494; E-mail: m.balfaki@alumni.sbu.ac.ir
Abstract: Regenerative medicine is a field of pharmacy and medicine that focuses on stem cells and other methods such as nanoscience and biotechnology to stimulate the body's natural regenerative processes and repairing damaged tissues and organs to improve function and reduce pain. In this review article, focus is on Dental Stem Cells (DSC) and other cells regeneration in human body. The appropriateness of tissue-engineered therapies relying on the multipotent regenerative abilities of DSC is accompanied by significant challenges, as growth factors and epigenetic components are crucial for preserving their multipotency while being susceptible to a range of natural and environmental factors. Current evidence highlights the positive outcomes associated with select regenerative therapies; nevertheless, to provide further support, additional data must be gathered through standardized therapies and further studies. Organoids (3D cell culture) and nano scaffolds are also being explored as potential tools for regenerative therapies. Understanding the mechanisms that determine the behavior of these cells and how they interact will enable future generation therapies. Demonstrating promise, cell therapy is an alternative approach within regenerative medicine. Developmental factors like extracellular vesicle production are thought to mediate the regenerative response through paracrine effects in cell therapy, which is widely recognized.
Introduction :
Regenerative therapies represent a promising approach in medical biology that utilizes gene tools and technologies to possibly cure a wide range of human diseases, maladies, and disorders remotely. Additionally, we have several regenerative treatments, such as stem cell therapy 1 which uses stem cells or their products to encourage the healing mechanism of damaged, diseased, or dysfunctional tissue. Tissue engineering demonstrates numerous areas to care for cancer, storage cord blood and cell banking, gastrointestinal and gynecology, dermal, dentistry, urology, musculoskeletal, orthopedics, spine, cardiology, vascular and neurology, and the subsequent failure in organ transplantation. It also uses cells rather than donor organs 2. The next method for regeneration therapies in biomedicine is gene therapy 3. By replacing diseased genes with viable duplicates and deactivating non-functioning harmful genes, the strategy involved in modifying a person’s genes for treating or curing diseases aligns seamlessly with the breakthroughs achieved in gene therapy. The next new approach in this field is Platelet-Rich Plasma (PRP) Therapy 4. The therapy is based on a designed process that modifies an individual’s genes to provide treatment or remedy for diseases that can synergize with gene therapy advancements through the substitution of a disease-causing gene with its healthy counterpart and the neutralization of an ineffective disease-causing gene 5.
Another therapy to regenerate the human body is Extracorporeal (outside the body) Shockwave Therapy (EWST) 6. A generation which makes use of surprise waves to deal with persistent painful situations of the musculoskeletal system. The process was spearheaded by the creation of EWST technology alongside the integration of fundamental physical-theoretical knowledge. An assortment of application devices such as electrohydraulic, piezoelectric and both flat and cylindrical electromagnetic focusing mechanisms were developed along with radial ballistic devices. Early tests were conducted to evaluate their performance in various applications 7. The last method of regeneration refer is Regenerative Rehabilitation 8. The end objective of regenerative medicine is to create novel and efficient approaches that enable tissue regeneration and repair, thus promoting functional restoration 9.
In this review, stem cell therapy for regeneration for dental disorders was studied with focus on some techniques and applications to repair and restore lost functions in tissues. The implementation of dental stem cell-based regenerative treatments involves the utilization of Dental Stem Cells (DCSs), 3D cell culture, biomaterials, signaling pathways, epigenetic direction, and immunomodulation. These components work together to advance the regeneration of dental tissues and move forward in dental care. Advanced exploration and advancement in these domains are crucial to unlocking the complete potential of DSCs in regenerative healthcare applications.
Stem cell research in dental tissues: DSCs are pluripotent cells that have the ability to continuously divide and produce pups that differentiate into several other cell types or tissue types. Adult solid teeth's dental pulp tissue has been isolated. Additionally, deciduous teeth are another promising area of research, offering exciting possibilities for regenerative dentistry applications 10.
Here are a few keys on DSCs: The confinement of DSCs includes extraction from different tissues within the teeth and encompassing structures:
1. The ability of DSCs like Gingival Mesenchymal Stem Cells (GMSCs), Dental Pulp Stem Cells (DPSCs), and Periodontal Ligament Stem Cells (PDLSCs) means they can clone and regenerate themselves which is extraordinary. This implies a method by which the stem cells isolate themselves to produce more stem cells, lifelong reproduction of the stem cell pool as well as multipotent isolation capacity and flexibility 11. The most recent study demonstrated the characteristics of these cells, including their ability to exhibit immunomodulatory and stem cell-like pathways and to promote tissue regeneration or recovery 11,12.
2. Dental stem cells (DSCs) have demonstrated significant potential in treating a variety of dental conditions, including periodontitis, dental caries, and Temporomandibular Joint (TMJ) osteoarthritis 13,14. They have to be investigated for potential in tissue engineering and regenerative medication application 15.
3. Regeneration of damaged dental tissues: Stem cell-based approaches are being explored to recover harmed or lost tissues within the verbal and maxillofacial locale, counting teeth 16. As figure 1 indicates the innovative organ germination strategy is utilized in regenerative treatment to effectively transplant bioengineered dental embryos comprising epithelial and mesenchymal cells, resulting in the complete recovery of functional teeth and bio-roots in vivo. Analysts are investigating procedures utilizing DSCs, biomaterials, and particular 3D culture conditions to imitate the characteristic specialty of DSCs. This approach points to addressing embedded disappointment and giving elective regenerative pharmaceutical alternatives for dental wellbeing 16,17.
4. Clinical applications: Clinical applications of DSCs have been utilized for treating dental ailments and surgical sites. They have the capability to recover harmed or lost tissues within the verbal and maxillofacial locale, counting teeth 18. Clinical trials are currently underway to evaluate the suitability and safety of DSC therapy for various dental applications 16. Their special characteristics, restorative potential, and capacity to recover harmed tissues make them important for the treatment of dental maladies and tissue designing applications. Progressing inquiries about clinical trials are growing our understanding of DSCs and some of their applications in dental care.
Also, for regenerative treatments without any immune system reaction from the body’s cells, DSCs can serve as a hopeful replacement for bone marrow-derived stem cells for inflammation. They have regenerative potential and can separate into different cell sorts included in tooth and tissue regeneration. DSCs are effectively available and helpful together, making them an appealing alternative for regenerative capacity dentistry applications. Continuous inquiry about points to optimize their regenerative capacity and create inventive procedures for dental tissue designing and regeneration process.
Distinctive types of dental stem cells and their properties: Recent research has identified and characterized several distinct types of dental stem cells. These cells exhibit unique characteristics and hold enormous promise for the field of regenerative medicine and the mechanism of cell metabolism. It is fascinating to observe the extraordinary capabilities of DSCs like DPSCs, Stem cell Human Deciduous (SHEDs), PDLSCs, Stem Cells collected from Apical Papilla (SCAPs), and Dental Follicle Progenitor Cells (DFPCs) within the domain of regenerative medicine as they continue to be identified and characterized. These cells possess invaluable characteristics like high stemness properties, regenerative activities, and a remarkable potential for differentiating into various cell types; thus, making them exceedingly favorable for advancements in tissue engineering and regenerative medicine in vivo 19,20. Based on recent search results, there are several potential advantages associated with utilizing DSCs in regenerative medicine including non-invasiveness, high stemness properties, regenerative activities and differentiation potential 20,21.
Here are some types of DSCs and their unique characteristics listed below: Dental pulp stem cells (DPSCs): DPSCs are a promising form of DSCs that can be isolated from the tooth's pulp considering they have characteristics similar to those of stem cells, including the ability to self-renew and divide into several cell types. In addition, they demonstrate immunomodulatory properties in an in vivo situation 22. DPSCs have a few properties and characteristics that make them profitable in different investigations and applications. Here are a few key properties of DPSCs based on the high proliferation rate 23, low immunogenicity 24, multipotency 24, differentiation potential 25, neuro-immunomodulatory properties 26, and angiogenic potential 27. It's critical to note that these properties may change depending on the particular thinking about or inquiry about the setting. DPSCs proceed to be a dynamic range of inquiries about, and investigations are underway to delve into the extensive possibilities and uses in regenerative medicine and tissue engineering.
Stem cell human deciduous (SHED): SHED, occupies a special position because they can be extracted from the pulp of milk teeth. These cells exhibit clonogenicity, the ability to self-renew and the ability to differentiate into many cell types. In addition, SHED exhibits both stem cell-like properties and immunomodulatory properties 28. SHED has a high proliferative rate and can separate into osteoblasts, neural cells or adipocytes and odontoblasts 19. Different development variables, calculations related to vascular endothelial development and figures reflecting platelet-derived development and fundamental fibroblast development calculations, had been investigated as natural signals to control stem cells' destiny for pulp regeneration 29,30.
The term (PDLSCs) designates the stem cells found in the periodontal ligament: PDLSCs are a type of dental stem cell that can be disconnected from the periodontal ligament 31. The perivascular space of the periodontium serves as a habitat for this stem cell, which has the potential to transplant and rejuvenate the Periodontal tendons (PDL). By using stem cells, growth factors or the extracellular skeleton, it becomes possible to effectively repair damaged periodontal tissue through tissue regeneration 31,32. Conjointly they can recognize different cell sorts included inside the course of action and back of the periodontium, which joins the gums, periodontal tendon, cementum, and alveolar bone 31,33. PDLSCs have been compared to other sorts of stem cells collected from Apical Papilla (SCAP) and deciduous teeth (SHED) and represent prime examples of diverse stem cell sources to evaluate their periodontal properties and separation potential 32. The impact of various factors, including Bone Morphogenetic Proteins (BMPs), commonly known as bone morphogens, has been explored 34.
Specifically, dental follicle stem cells (DFSCs): DSCs can detach from tooth follicles. These particular cells exhibit clonogenicity and self-renewal abilities while possessing multipotent separable abilities. In addition, they exhibit both stem cell-like properties and immunomodulatory properties 35,36. The presence of pluripotency markers like OCT-4, SOX-2, and NANOG in DFPCs demonstrates their self-renewal capacity and multipotent potential 37. DFSCs can attain osteogenic, adaptogenic, chondrogenic, neural, and cardiomyocytic separation in a particularly initiated environment 38. DFSCs outperform other DSCs by having a much higher rate of cell expansion, greater ability to form colonies, as well as a more primitive and much better anti-inflammatory effect 35.
Table 1 details the classification and research progress of Dental Stem Cells specifically in relation to periapical lesions and their regeneration 45,46.
There are several FDA approvals that involve the use of DSCs or stem cell-derived technologies. Some examples of such notable uses follow:
AlloDerm Regenerative Tissue Matrix is an acellular dermal matrix derived from donated human skin, used in various dental and surgical procedures. It finds its application in periodontal surgery and grafting for its inherent property of tissue regeneration. Though not a stem cell product, it is mixed with DSCs to enhance the healing and regeneration process in oral applications.
Another example of FDA clinical approval is Osteogeny which is a bone grafting material that can be used in conjunction with stem cells. It finds its application in dental implant procedures for the purpose of encouraging bone regeneration. If combined with DPSCs, the osteogenic potentiality is enhanced, hence it can serve as a tool in regenerative dentistry.
The Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) are obtained from the patient's blood and contain growth factors responsible for promoting healing. These products have been commonly used in the enhancement of a regenerative process in periodontal and implant surgeries in conjunction with DSC therapies. Though they themselves are not FDA-approved as direct stem cell therapies, their application with stem cells is immense in clinical practice 47-49.
Stem cells originating from the dental apical papilla, also known as (SCAPs): Mesenchymal stem cells, so-called SCAPs, obtained from the apical papillae of young teeth have great potential for regenerative endodontic applications 31,50. The results of a recent comparative study examining DPSC and SCAP indicate that the two cell types have different mesenchymal stem cell markers and may have different genetic backgrounds. Although no significant differences in cell viability, growth potential, or motility were observed between DPSCs and SCAP, discrepancies in the emitted profile were observed 50 and the remarkable differentiating abilities of DSCs as shown in figure 2. A recent study comparing DPSCs and SCAPs discovered that both cell types expressed mesenchymal stem cell markers and exhibited the potential to differentiate into different lineages. Although no significant differences were observed in terms of cell functionality, proliferation, or motility between DPSC and SCAP, variations were noted in the secretion profile.
Through analysis, it was shown that the fusion of Mineral Trioxide Aggregate (MTA), Enamel Matrix Derivative (EMD), and Low-level laser therapy (LLLT) complemented by dentine generated notable consequences. EMD and LLLT seem to lead to a critical increment in SCAP practicality compared with another treatment bunch 51. This study aimed to investigate the properties of human Dental Pulp Stem Cells (hDPSC)-derived, Jagged1-treated dECM (Extracellular Matrix) and its intrinsic effect on odonto-/osteogenic SCAP separation, leading to the discovery of dECM-like fibrous organizational structures. The effects of decellularized ECM is the next important finding from recent research on SCAPs. There was a reduction in the components of calcium and phosphate following deECM 52. Another investigation demonstrates the SCAP features' cytokine profiling. The key goal of this study is to examine the effects of oral bacteria and their supernatants on the cytokine profile of SCAP, with particular attention to the potential consequences for osteogenic and immunogenic characteristics 53.
The objective of this study is to investigate the effects of oral bacteria and their supernatants on the SCAP cytokine profile and evaluate how they may influence the osteogenic and immunogenic potential of SCAP included in tooth arrangement and repair. They have the potential to be used in regenerative dentistry procedures such as dental pulp, periodontal tissue, and dental tissue regeneration. The potential of these DSCs in treating dental diseases and restoring oral health is being continuously explored through ongoing clinical studies.
Organoids (3Dcel culture) used in dental stem cell research: Organoids are three-dimensional (3D) systems for cell culture that imitate the composition and capabilities of human organs. They are created from stem cells. They offer a more physiologically appropriate habitat closer to in vivo circumstances, which substantially improves over typical two-dimensional (2D) cultures. Because of their capacity to facilitate more accurate modelling of human tissues, organoids are useful in a number of scientific categories, including drug development, personalized medicine, and cancer research 54. Within the final decade and recently researched, organoid innovation has shown to be a useful tool for studying tissue science and the advancement of tissue transplanted in a 3D extracellular matrix platform throughout the last ten years of research 55. Organoids offer scientists an exciting opportunity to study signaling pathways and edit genomes in a body-like environment. However, they also have several limitations that mimic real-life challenges 56,57.
Nano scaffold are designed to mimic the natural extracellular matrix, providing structural support for cell attachment, growth, and differentiation that simulates the Extracellular Matrix (ECM), like Matrigel, is used to seed the distinct essential tissue test, which contains stem cells either as single cells or enclosed within cell clusters. These developments are characterized by a multidisciplinary approach that combines engineering principles with biomedical sciences, resulting in improved medical equipment 54. The scaffold is subsequently modified using a specific combination of developmental components that simulate stem cell specialization 58,59. In addition, organoids can be employed to examine the interplay between stem cells and immune cells, encompassing the function of mediators/cytokines in simulated conditions 60,61. Researchers may learn more about how different organs form and function in certain areas, such as the oral and maxillofacial tract, by using organoids as experimental models. These microscopic systems also have enormous promise for disease modelling, which may help with medication discovery and provide insight into the relationships between hosts and pathogens. Additionally, they provide incredible chances for studying the complexities of organogenesis and regenerative medicine 62. Furthermore, organoids exhibit the capacity to replicate an important proportion of the complexity, unique characteristics, and genetic markers found in the original tissues and organs 62. Organoids are 3D cell culture systems used for basic science, medicine, and drug development that replicate in vivo environments. DSC can be used in bone and dental tissue engineering, and they show great promise for tissue regeneration 63.
Overcoming the limitations in the availability and culture-ability of epithelial stem cells from essential tissue, serial extension (passaging) of organoid societies provides an invaluable and reliable source, while minimizing any negative traits that may be present 64. The construction of epithelial organoid models is typically performed without prior segregation of the epithelial (stem) cells from the extracted tissue sample, as a result of mesenchymal cells being unable to thrive within the specific culture conditions employed and being readily discarded 65. Organoids are three-dimensional (3D) cultures that have been utilized in DSC inquiries to investigate tooth-specific science 66. This occurs when cells or tissue fragments, such as stem cells, are implanted into a three-dimensional scaffold within the extracellular matrix 55.
Some preferences for utilizing organoids in DSC research are as follows: DSCs have the ability to summarize the complex structure of tissue, making them organoids, as compared to traditional 2D cell cultures, provide a more physiologically significant exhibition. They can replicate the intricate engineering and biological intuition present in healthy tissues, enabling analysts to consider tooth-specific science and improvement with greater precision 66,67. They can demonstrate genetic control, allowing for the hereditary modification of organoids and allowing analysts to consider the effects of specific traits on tooth development and function. This makes it possible to investigate the inherited factors associated with oral diseases and possible beneficial targets 68.
Long-term culture and development are the main parameters in DSC culture so organoids can be extended and kept up in culture for expanded periods, permitting long-term consideration and experimentation. This empowers analysts to observe the development and behaviour of DSCs over time 67. Figure 3 shows following the extraction of hDPSCs from T75 flasks, they were seeded into 24 well plates for two-dimensional culture, with subsequent transfer into drop formations on 24 suspension cell culture plates.
Patient-derived organoids can be utilized to study personal varieties in tooth improvement and illness. This permits personalized pharmaceutical approaches, where medications can be custom-made to an individual's particular hereditary cosmetics and characteristics 16.
Organoids be used to study dental diseases and disorders: Organoids are three-dimensional (3D) structures that can model the architecture and function of human organs. Pluripotent stem cells, adult stem cells, or somatic cells serve as a source for them and can self-organize to reproduce the complexity of many tissues. Such self-organization allows the maintenance of key functional, structural, and biological properties similar to the original organ they represent 64,69.
Organoids can be utilized to ponder dental diseases and disorders. Organoids give a 3D culture framework that permits complex intuition between cells, the stream of flagging atoms and supplements, and self-organization, which makes a difference in researchers demonstrating and getting dental physiology 70,71. Organoids can be created by using cells that have been taken from individuals with particular dental conditions, such as jaw and facial cancer and dental dysplasia 72. This permits analysts to imitate the infection in a controlled research facility setting and think about its components and movement 70. Also, organoids can be utilized to analyze the morphological and useful changes related to salivary organ dysfunctions, such as pathobiology, inflammation, and regenerative pharmaceuticals 73.
Organoids have moreover been utilized to study the intestinal epithelial barrier in fiery bowel maladies, and to address respiratory diseases, they have been generated from either endogenous lung epithelial stem/progenitor cells or pluripotent stem cells 74. Organoids derived from human tooth tissue have been created to investigate tooth epithelial stem cell science and epithelium-mesenchyme intelligence, Consequently, this breakthrough could lead to advancements in tooth-regenerative therapy as a viable option instead of relying solely on synthetic materials for implants 75. Organoids can be utilized to consider different dental diseases and disorders, including maxillofacial tumours, tooth dysplasia 70, and salivary gland dysfunctions 71.
Scaffold cell culture: The utilization of biomaterials as scaffolds aids in the growth and differentiation of dental stem cell: DSC-based regenerative treatments rely heavily on the use of biomaterials 76. They are utilized as scaffolds by producing nano polymers electrospinning 77 to support the development and diffraction of DSCs and cells within dental tissue have the remarkable capability to imitate the natural extracellular matrix. Figure 4 showcases a depiction in the form of a schematic diagram. This presentation highlights the seeding of DSCs on a hybrid scaffold and emphasizes their capability to differentiate, underscoring the potential for tissue regeneration in clinical applications, allowing for biodegradable and biocompatible properties that foster a conducive environment for the attachment, growth, proliferation, and differentiation of stem cells in a three-dimensional shape 78,79. Various types of biocompatibility and biodegradability biomaterials have been utilized in dental stem cell-based regenerative treatments as scaffolds, such as gelatin methacrylate (GelMA) hydrogel that create covalent bonds. These naturally derived materials are biocompatible and non-toxic to cells, allowing them to interact harmoniously with each other 80. Cell culture compatible, the biodegradable copolymers PGA/PLGA exhibit unique design capabilities and property 80 and collagen-nanoHA/OPS bio composite nano scaffolds provide a supportive structure for the DSCs growth and differentiation and showed a high rate of cell proliferation. The adhesion, migration, and spatial distribution of hDFMSC within the 3D matrix were enhanced by the increased deposition of extracellular bone matrix (osteopontin) and high ALP activity, suggesting early osteogenic differentiation. The existence of human OPN was the cause of this improvement 79,81,82.
It has been discovered and experimented that using engendered scaffolds made of Poly (ε-caprolactone) (PCL), Chitosan (CS), and MTA increases the expression of angiogenic markers in (DISCs). In order to promote the angiogenesis and osteogenesis of hDPSCs, scaffolds made of biopolymers from MTA/polycaprolactone (PCL) composites coated with Caffeic Acid (CA) that affected the physicochemical and biological features which were used 83. Successful results were obtained when researchers used 3D scaffolds containing MTA/polycaprolactone (PCL) composites coated with CA to stimulate angiogenic and osteogenic processes in hDPSCs 84. This may help DSCs differentiate more effectively into specific cell types, such as odontoblasts and cementoblasts 85. The possibility of using 3D porous chitosan scaffolds to treat spinal cord injuries is suggested by their capacity to induce neural development in hDPSCs 86.
Biomaterials can have a different organic direct effect on differentiation of DSCs in the human body. A few biomaterials can advance the multiplication and separation of DSCs, whereas others can inhibit their separation. By selecting suitable biomaterial coordinating the differentiation process of DSCs can lead them to develop into specific cell types 87. Mechanosensory pathways that regulate stem cell differentiation and separation to facilitate cell migration can be activated by them in DSC pulp. Gaining knowledge of these pathways can help improve the differentiation and multiplication of DSCs and thus can assist in the regeneration of tissue 85,87. Biomaterials can be falsely synthesized and designed to supply a steady structure for the development and the ability to differentiate DSCs leads to the generation of numerous quantities that are valuable in regenerative treatments 79,82.
Extraction of DSCs is made possible by the distinct developmental phases in regeneration observed in diverse dental tissues such as dental pulp, apical papilla, dental follicle, tooth germ, deciduous teeth, periodontal ligament, and gingiva 88,89. Their potential as an excellent bone platform material has led to the exploration of a wide variety of biomaterials, including polymers, ceramics, and composites 89.
What distinct categories of biomaterials are applied in dental tissue engineering: In dental tissue engineering, we have utilized distinctive types of biomaterials that are constructed from nano size to increase efficiency 90 as platforms to back the regeneration and development of dental cells and tissues in culture. Different groups can be formed based on the composition of biomaterials, properties, or applications to show the best performance in medical engineering. Here are a few of the distinctive types of biomaterials commonly utilized in dental tissue engineering 79.
For this model, we can use collagen a characteristic protein found within the extracellular matrix of different tissues, counting dental tissues. This substance stands out for its ability to be biocompatible, biodegradable, and possess remarkable cell adhesion properties. Collagen platforms can give structural back and advance cell connection, expansion, and differentiation 91.
Utilized in dental stem cell culture, poly(lactic) acid (PLA) is an example of a biomaterial. PLA is an artificial polymer that demonstrates both biodegradability and biocompatibility feature. Its mechanical qualities and ability to promote cell proliferation have led to its widespread use in dental tissue regeneration 91,92. One suitable biomaterial for use in DSC culture is hydrogel, which are composed of hydrophilic polymers properties in a three-dimensional configuration, have a large capacity to absorb and store water. Their features are similar to those of the common extracellular network to growth cells on it, and they offer an environment that is favorable to tissue regeneration and cell proliferation. For dental pulp tissue engineering, hydrogels such like fibrin and self-assembling peptides have been investigated in vitro 91,93. Due to the fact bioactive ceramics like hydroxyapatite and tricalcium phosphate can improve bone and dental regeneration, much research has been done on them. In addition to providing a scaffold framework, ceramics can release ions that promote tissue structure and promote cell activity 89. Considering they can be transferred minimally extensively, injectable biomaterials including hydrogels and nanofibers have gained attention in dental tissue engineering. These substances can be injected into the malformation area and used to frame as a scaffold there, supporting tissue regeneration region 94. There are several researches in the process of growing DSCs, a variety of biomaterials have been employed as scaffolds. The specific application and desired outcomes play a major role in determining the biomaterial selection in dental tissue engineering 95. Analysts are still researching and developing modern biomaterials with cutting-edge properties to increase the efficacy of dental tissue regeneration.
For instance, a clinical trial explored the use of nanostructured materials to deliver growth factors that promote the proliferation and differentiation of DSCs in situ. The outcomes suggested improved healing rates and tissue integration compared to traditional methods 27.
Research has shown that nanoparticles can improve the delivery and efficacy of DSC therapies by enhancing targeted drug delivery, such as growth factors responsible for tissue regeneration. Preclinical studies have proven this very effective; thus, translation into clinical trials is considered in order to evaluate its efficacy in human patients 96.
Compared with conventional materials, the production costs associated with integrating nanoparticles into dental applications are frequently greater. This is because creating and working with materials at the nanoscale requires specialized techniques that might be resource-intensive and call for cutting-edge technology. Innovation and economy of scale must be maintained. Although nanotechnology has tremendous potential for improving dental treatments, both patients and professionals are quite concerned about how affordable these cutting-edge technologies will be. For these advancements to be widely used, it is imperative that they be made accessible 97.
Nanoparticle-based therapeutics are intrinsically complicated because of their multicomponent nature. Designing and engineering such nanoparticles requires extra care and attention to detail, as even slight changes in composition can significantly impact the physicochemical properties and biological behavior of these particles. For example, reproducibility and consistency of the product characteristics during manufacturing are a great challenge, since most synthesis and characterization techniques applied for nanoparticles are sophisticated ones 98. The manufacturing complexities to arrive at appropriate liposomal formulations for drug delivery are multidimensional. For instance, the liposomes have to be engineered to realize defined sizes and surface properties that enhance drug loading and targeting efficacy. Variability in production processes may result in batch-to-batch inconsistencies, further complicating regulatory approval and clinical translation 99.
Quality control is crucial but difficult to ensure in the production of nanomedicine. Advanced analytical techniques are necessary for adequate nanoparticle formulation characterization. In addition, quality control measures common for conventional drugs may be inadequate, and testing methods have to be more sophisticated to evaluate not only physical but also biological properties of nanoparticles. For instance, such variability in the size and surface charge of nanoparticles may influence their therapeutic efficacy and safety profiles. Hence, the development of robust quality control protocols by using multiple orthogonal characterization techniques becomes essential for ensuring consistent product quality 98,99.
Signaling pathways culture in the regeneration of dental stem cell: Signaling pathways are significant within the laboratory's culture and the improvement in the comprehension and regulation of these pathways holds promise for enhancing the regenerative potential of dental pulp and contributing to breakthroughs in techniques for dental tissue regeneration. Signaling transduction pathways are included in regulating the regenerative potential of DSCs 100. Expound modulation of signaling transduction spatially and transiently through growth factors in dish and conveyance can advance basic and useful dental pulp regeneration 100. The pivotal component in the pulp DSC signaling pathway, ascertainable at the basal region of the developing dental root, is none other than the apical papilla. This structure contains multipotent stem cells that exhibit a remarkable capacity for diverse differentiation processes 101. Another factor in the signaling pathway is cell-cell communication which plays a critical part in DSC culture regeneration. Dental Mesenchymal Stem Cells (DMSCs) have appeared to deliver a secretum, counting solvent factors and extracellular vesicles, that can initiate paracrine activity and advanced tissue regeneration 102. During the expansion phase, it has been noted that cell-cell communication involving STAT3/Oct-4/Sox2 signaling enhances the pluripotency of dental pulp cells and periodontal ligament cells, making them highly favourable for dental tissue regeneration 103.
The establishment of protocols for isolation, cryopreservation, and quality control has facilitated the utilization of cryopreserved stem cells derived from dental tissue 104. In addition, scientific studies have revealed that DPSCs hold the capacity to develop into useful neurons in controlled environments, showcasing their potential role in cellular treatments for neurodegenerative disorders 105.
DSCs and their secretions have shown promise for tissue regeneration, and advanced research appears to be targeting their components of cell-to-cell communication. There are, diverse signaling pathways that influence DSC differentiation in different ways (Table 2) 88. Understanding these pathways can offer assistance in creating novel techniques for dental tissue regeneration. One instance is cytokines or developmental substances like Stroma cell-Derived factor (SDF), Fibroblast Growth Factor (FGF), BMP, and Vascular Endothelial Growth Factor (VEGF) by itself, Wnt can individually stimulate DSCs in terms of movement, proliferation, odontogenic differentiation, pro-angiogenesis, and pro-neurogenesis potentials 100. Another signaling pathway is the STAT3/Oct-4/Sox2 signaling pathway, through STAT3/Oct-4/Sox2 signaling, the co-culture systems demonstrated a substantial enhancement in the expression of Oct-4, Sox2, and STAT3, leading to improved dental pulp cells and periodontal ligament cells via cell-cell communication 103.
Immunomodulating factor in DSC for regeneration: DSC culture exhibits an exceptional feature known as immunomodulation that can contribute to making strides in their viability in dental regenerative medication 110. In the treatment of neurodegenerative diseases, neural injuries, cartilage defects, and bone repair, the DPSC-derived secretum demonstrates efficacy due to its ability to regulate neuroprotective, anti-inflammatory, antiapoptotic, and angiogenic processes with its assortment of biomolecules 111. The inborn properties of self-renewal, immunomodulation, proangiogenic potential, and multilineage power have intrigued DSCs for their application within the regeneration of dental tissues 112. DPSCs have been employed in the dental field in a novel approach to treat oviduct wounds through DPSCs-based therapy through immunomodulation and angiogenesis in vivo 113. While inflammation is an essential component of wound healing, an excess of it may cause damage to tissues and hinder the healing process. Immunomodulating factors are instrumental in regulating inflammation by altering macrophage behaviours and suppressing the release of proinflammatory cytokines 114. It has been revealed that the microbiota that inhabits the human body could be the modulator of MSCs, including DSCs, as well 115.
Understanding the interactions between microbiota and DSCs is crucial for making significant advancements in the field of Regenerative Medicine 115. Later investigations have looked for ways to optimize DSC functionality including the ability to work in vitro and in vivo, preparation of culture conditions, exposure to mechanical and physical stimuli, preconditioning by cytokines and growth variables, and genetic modification of dental stem cells 112,116. Using growth factors such as SDF, FGF, BMP, VEGF and Wnt, DSCs can efficiently regenerate lost pulp through mechanisms such as increased migratory capacity and increased proliferation and differentiation potential. The extracts obtained from DPSCs contain a diverse array of biomolecules. These extracts have shown effectiveness in treating neurodegenerative diseases, nerve damage, cartilage defects, and bone repair by modulating neuroprotective, anti-inflammatory, anti-apoptotic, and angiogenic mechanisms 100.
Immunomodulatory mechanisms in dental stem cells: a pathway to enhanced regeneration: Nanomaterials for DSC regeneration provide a long-term opportunity as well as a challenge. On one hand, their use can improve regenerative outcomes, but on the other hand, their interactions with the immune system and possible effects after long exposure have to be taken into consideration. Further studies will be necessary to relate these interactions and enable nanotechnology to be safely translated into dental clinical practice. According to the British Journal of Cancer in 2022, the long-term follow-up has been higher in clinical trials; according to them, many toxicities take years to show up. Presently, this stands as a wide perspective for therapies that involve either the stem cells or the nanotechnology treatment, thereby establishing that in all these therapies; patients need to be followed up over time due to the probability of capturing complications arising much later 117. Although preliminary results are encouraging, there is a pressing need for more long-term studies that assess the interactions of these therapies with biological systems over an extended period, concerning complications such as chronic inflammation or tumor formation 118. For example, engineered nanoparticles might be perceived as harmful by the immune system, causing an unintended immune response that could compromise their therapeutic efficacy. The biomolecular corona concept refers to how, upon entrance into the body, proteins adsorb onto nanoparticles, which may alter their biological identity and immune responses. This interaction can either enhance or suppress immune recognition and response. Understanding this phenomenon becomes important for designing safer nanomaterials for dental applications 119.
Cytokines and growth factors are gearing up to enhance MSCs' abilities: Cytokines, in addition to developmental variables, can influence tissues through paracrine effects on MSCs. Cytokines can interact between development components by their receptors on the surface and dynamic signal cell survival. Migration and differentiation still take place despite the repression of T cell and B cell activation, as well as dendritic cell differentiation, while simultaneously impairing the cytolytic potential of Natural Killer (NK) cells and enhancing regulatory T cell differentiation 120. In any case, the immunomodulatory impact of MSCs isn't continuously accomplished but requires incitement by provocative variables, cells can be influenced by various substances, including IFN-γ, TNF-α, and IL-1β 121. cytokine preparation is recommended to be a supplemental atomic procedure to cultivate the helpful potential of MSCs and contribute to building up an affable microenvironment for dental tissue repair. Specifically, dental MSC-derived secretum/conditioned medium, which contains bioactive particles Exhibiting attributes like anti-inflammatory, antiapoptotic, angiogenic, osteogenic, and neurogenic effects, these arbiters propose an exceptional cell-free regenerative and therapeutic strategy that surpasses parent cells in various aspects 112,122.
The research findings indicate that cytokines and growth factors can play a role in improving the effectiveness of mesenchymal stem cells (MSCs) in regenerative medicine: below shows the ability of cytokine and growth factors:
1. Pulling MSCs towards the injured area: The engineered MSCs utilize the CXC chemokine receptor 4 (CXCR4)-stromal cell-derived factor 1α (SDF1α) axis to migrate towards the site of injury, promoting vasculogenesis through the secretion of cytokines and growth factors by paracrine secretion and improved neuroprotective approaches to optimize supply, foster recovery of axon connectivity, and boost behavioural ability 123.
2. Extraordinary cytokine profiles: The therapeutic potential of stem cells from SHED and DPSCs may be influenced by their unique cytokine profiles 19.
3. The significance of MSCs preconditioning: Adapting MSCs before therapeutic use by exposing them ex vivo to hypoxia, inflammatory stimulus, or alternative factors/conditions is a custom strategy designed to enable MSCs survival in hostile surroundings and amplify their role in regulating local immune responses 124.
4. The paracrine effect is observed: The cytokine production capability of MSCs facilitates their role in regulating the immune microenvironment and stimulating the repair process of endothelial cells, fibroblasts, and tissue precursor cells in wounds such as the cytokine interleukin-6 (IL-6), the chemokine CCL2, the angiogenic factor VEGF, and the FGF 125.
Discussion :
Regenerative therapy is a specialization of pharmacy and medicine. It mainly focuses on the employment of stem cells, besides other modalities like nanoscience and biotechnology, to stimulate intrinsic regenerative procedures of the body, hence restoring functionalities to damaged tissues or organs. It is also used in improving function and pain following injury. In this review article, we have focused on DSCs for the regeneration of dental cells and other cells in humans. The appropriateness of tissue-engineered therapies relying on the multipotent regenerative abilities of this cells is accompanied by significant challenges, as growth factors and epigenetic components are crucial for preserving their multipotency, while being susceptible to a range of natural and environmental factors. Current evidence highlights the positive outcomes associated with select regenerative therapies; nevertheless, to provide further support, additional data must be gathered through standardized therapies and further studies. Organoids (3D cell culture) and nano scaffolds are also being explored as potential tools for regenerative therapies.
Strategies based on nanotechnology are being investigated to improve the safety and effectiveness of stem cell treatments for dental regeneration. Although they are still in early stages, there are several crucial clinical uses. To assess their safety, effectiveness, and clinical transferability for everyday dental uses, further extensive research and clinical trials are required. Widespread clinical acceptance will depend on overcoming obstacles with regard to immunological rejection, regulatory permission, and the source of stem cells. Future generations of respiratory therapeutics will be made possible by an understanding of the mechanisms governing these cells' behavior and interactions with their habitats. Cell therapy is an alternate strategy in regenerative medicine that shows potential. It is well known that developmental factors such as cytokine parameters and extracellular vesicle formation are expected to mediate the regenerative response in cell therapy through paracrine actions. Although clinical trials have shown stem cell therapy to be safe, it has the potential to change into illness. In contrast, stem cells and nanotechnology have great potential to improve dental regeneration and treatment; there are substantial ethical and legal barriers that must be addressed before these technologies can be fully utilized. The area of regenerative dentistry is quickly developing, and when new tools and methods appear, it is critical to implement safety measures to guarantee their responsible and ethical application.
However, considering the promising results from animal models and early-phase human trials, dental applications of these approaches are still lacking profound long-term data concerning efficacy and safety. Possible risks of tumorigenesis and immune responses from the manipulation of stem cells demand a lengthy follow-up period in clinical studies to confirm such modalities.
Ethical concerns on the source of stem cells are significant in DSC therapy, whereby one has to distinguish between embryonic and adult stem cells. This discussion encompasses the moral implications of sourcing these cells, as well as the strategies implemented to address these challenges. For instance, the use of embryonic stem cells has raised deep ethical concerns mainly because human embryos must be destroyed to acquire these cells. Critics maintain that this act is a violation of the moral status accorded to embryos, which a number of people argue should be accorded protection as a form of human life. This is a contention that evokes heated debates on personhood and the ethical use of embryos for research and therapy. Obtaining informed consent from the donors is essential but complex, since many people attach emotions and morals to reproductive materials. In contrast, adult stem cells, including those from dental pulp and other tissues, generally pose fewer ethical issues. These could normally be retrieved with less moral complaint, as no embryo destruction is involved in the process.
The most common sources for adult stem cells are bone marrow, adipose tissue, and umbilical cord blood. These have become widely accepted sources both in research study and clinically, as they do not raise the same ethical dilemmas as embryonic stem cells.
The collection and use of adult stem cells are governed by established regulations that ensure respect for informed consent and donor rights, thereby reducing the risk of exploitation or coercion. Setting standardized guidelines on stem cell research may ensure that ethical issues are considered within the framework of the study. The guidelines should cover best practices in sourcing, handling, and utilization of the stem cells, hence encouraging responsible research. Public transparency should be improved through public information and education on stem cell research. These would include open forums, workshops, and clearly available resources that explain the science supporting stem cell therapies, the ethical issues involved, and the potential benefits. Engaging various stakeholders will help build trust and understanding. Efforts must be directed to make any progress with stem cell technologies available to diverse populations. This would relate to disparities in access to healthcare and the affordability of treatments created through stem cell research. Strategies may involve policy advocacy for equitable healthcare distribution and support for underserved communities.
Conclusion :
DSCs present a viable path for regenerative medicine and regeneration. These cells are notable for having intriguing characteristics such as multipotent separable ability, clonogenicity, and self-renewal that make them appropriate for tissue regeneration. Organoids are three-dimensional (3D) communities that have been utilized to reflect tooth-specific science and regenerative medicine in the field of DSCs research. They offer a valuable resource for researching prospective tooth regeneration, learning about tooth homeostasis, and researching human dental stem cell science. Nano scaffolds, cell treatment, signaling pathways, and epigenetic and immunomodulating approaches are efforts made to explore and improve the regenerative potential of DSCs. Numerous inquiries and articles have discussed the potential use of DSCs in tooth repair, reclamation, and recovery through tissue engineering and regenerative pharmaceutical applications. DSCs have the potential to repair and regenerate dental pulp, periodontal tissue and other oral structures. DSCs have to be examined for their great potential for non-dental applications, such as the advancement of stem cell-based solutions for heart, brain and bone repair. We also found that components such as epigenetics and immunological impacts as well as development variables in tissue engineering play a principal part in the development and differentiation of these cells. To conclude, DSCs emerge as a promising strategy for advancing regenerative medicine in both dentistry and its broader applications. The rising demand for regenerated materials fuels ongoing clinical trials.
Conflict of Interest :
Authors declare no conflict of interest.
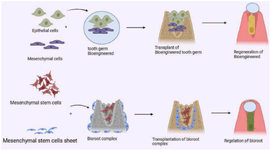
Figure 1. Utilizing an innovative organ germination strategy, regenerative treatment facilitates the transplantation of bioengineered dental embryos composed of epithelial and mesenchymal cells to restore fully functional teeth and bio-roots in vivo.
|
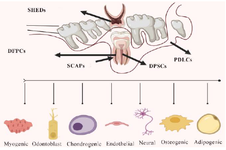
Figure 2. Dental Stem Cells (DSCs) particularly Dental Pulp Stem Cells (DPSCs) and Stem Cells from Apical Papilla (SCAPs) exhibit significant differentiation potentials that are being explored for regenerative medicine applications, especially in dental tissue engineering.
|
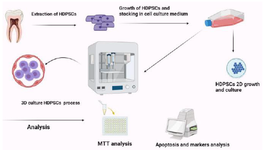
Figure 3. Extraction of a third molar involved a procedure that assured aseptic conditions during the removal of its pulp. Through collagenase digestion, the extracted pulp tissue provided suitable material for growing human Dental Pulp Stem Cells (hDPSCs) employing seeding. These cultured hDPSCs underwent passaging in T75 flasks for utilization in 3D culturing experiments and future cell stocks. Following the extraction of hDPSCs from T75 flasks, they were seeded into 24 well plates for two-dimensional culture, with subsequent transfer into drop formations on 24 suspension cell culture plates. Following a 4-day growth period, hDPSCs were collected from all scenarios including the 2D and 3D medium, and various analyses were carried out.
|
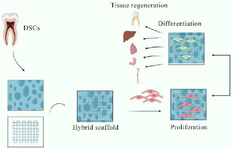
Figure 4. A depiction in the form of a schematic diagram exhibiting the seeding of DSCs on a hybrid scaffold and highlighting their differentiation capability is presented, emphasizing the potential clinical applications for tissue regeneration.
|
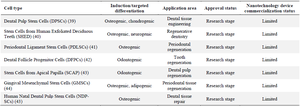
Table 1. Dental stem cells, also known as Dental Pulp Stem Cells (DPSCs), are a promising source of regenerative medicine due to their unique properties and ability to differentiate into various cell types
|
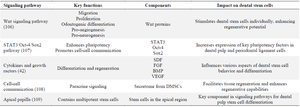
Table 2. Comparing the various signaling pathways involved in the regeneration of dental stem cells
|
|