The Protective Effect of N-acetylcysteine against Deltamethrin-Induced Hepatotoxicity in Mice
-
Ameri, Ali
-
Student Research Committee, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
Rahmati, Alireza
-
Student Research Committee, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
Soroushfar , Shadi
-
Student Research Committee, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
Lalehzari, Mehdi
-
Trauma Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
Dehghani, Tahereh
-
Infectious and Tropical Diseases Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
Haghi-Aminjan, Hamed
-
Pharmaceutical Sciences Research Center, Ardabil University of Medical Sciences, Ardabil, Iran
-
Shamseddin, Jebreil
-
Infectious and Tropical Diseases Research Center, Hormozgan Health Institute, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
-
 Omidi, Mahmoud
Endocrinology and Metabolism Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran, Tel: +98 9189407031; Fax: +98 76 33710405; E-mail: toxicology@hums.ac.ir
Omidi, Mahmoud
Endocrinology and Metabolism Research Center, Hormozgan University of Medical Sciences, Bandar Abbas, Iran, Tel: +98 9189407031; Fax: +98 76 33710405; E-mail: toxicology@hums.ac.ir
-
Department of Pharmacology and Toxicology, Faculty of Pharmacy, Hormozgan University of Medical Sciences, Bandar Abbas, Iran
Abstract: Background: Exposure to pesticides is of concern to public health officials worldwide. Deltamethrin is a synthetic pyrethroid pesticide which is widely used in agriculture and veterinary medicine. Deltamethrin poisoning is always one of the concerns in medical centers due to the deltamethrin induced hepatotoxicity. This study evaluated the hepatoprotective effects of N-acetylcysteine (NAC) against deltamethrin induced hepatotoxicity in mice
Methods: A total of 40 BALB/c male mice were randomly divided into four groups; the first group was used as a control (0.5 ml normal saline); Groups 2-4 were treated with NAC [160 mg/kg Body Weight (BW)], deltamethrin (50 mg/kg BW), and NAC plus deltamethrin. At 1 and 24 hr after treatment, the animals were sacrificed and blood and liver samples were obtained for analysis and the liver/body ration, hepatic enzymes as Aspartate aminotransferase (AST), Alanine Transaminase (ALT), Alkaline phosphatase (ALP), Lactate dehydrogenase (LDH), Glutathione (GSH) content and Reactive Oxygen Species (ROS) level were measured. For comparison between more than two experimental groups, one-way ANOVA following Tukey test was used by SPSS software.
Results: The deltamethrin significantly increased AST, ALT, ALP, and the level of ROS level at the end of 1 and 24 hr after treatment; while the LDH level and GSH content were decreased. Mice in the deltamethrin treated group had a higher liver/body weight ratio than in other treated groups after 24 hr. On the other hand, NAC in combination with deltamethrin significantly reduced the activities of AST, ALT, ALP, and increased GSH levels.
Conclusion: This study demonstrated that NAC has a hepatoprotective role against deltamethrin-induced toxicity.
Introduction :
Deltamethrin is the most common pesticides from pyrethroid class of insecticides. Nowadays, deltamethrin is widely used as a pesticide in agriculture and easily available 1. Deltamethrin despite its excellent pesticidal effects, is highly toxic liver inducing hepatotoxicity which could to death 2. Sometimes people abuse deltamethrin for suicide 3 or to poison others 4. For this reason, the study aimed to find an antidote to deal with deltamethrin poisoning.
Study on farm workers in Karachi, Pakistan showed that in those who were exposed to the deltamethrin pesticide, hepatic enzymes such as serum Glutamic Oxaloacetic Transaminase (GOT), serum Glutamic Pyruvic Transaminase (GPT), and Alkaline Phospha-tase (ALP) were dangerously elevated 5. Another study showed that farmers who were exposed more to the deltamethrin pesticide had more involvement of liver cirrhosis in different stages 6. Another study in India showed that farmers who were exposed to the deltamethrin pesticide had significantly more infected liver diseases and increased levels of bilirubin in their blood 7. The study conducted in 2020 on farmers who were exposed to deltamethrin pesticide showed that farmers exposed to deltamethrin had significantly more liver damages 8.
N-acetylcysteine (NAC) is the acetylated form of the amino acid L-cysteine used as a mucolytic agent and is available in the pharmaceutical market 9. N-acetylcysteine contains a thiol group that can interact with and neutralizes free radicals such as Reactive Oxygen Species (ROS) and nitrogen species 10. N-acetylcysteine can also increase the amount of reduced Glutathione (GSH) in the liver and therefore has beneficial antioxidant activities 11. Studies have shown that NAC, with its antioxidant capacity and increasing the amount of regenerative GSH in the liver, reduces oxidative stress and inflammation in the liver and prevents excessive liver damage 12. The study of rats exposed to organophosphate toxins has shown that NAC could prevent oxidative stress and inflammation caused by organophosphate toxins in rat liver 13.
According to the protective effects of NAC on the liver, it may be able to protect the liver against the toxic effects of deltamethrin. Therefore, in this study we aimed to investigate the protective effect of NAC against deltamethrin-induced hepatotoxicity in mice.
Materials and Methods :
Chemicals: Chemicals were obtained from the following suppliers: Sodium thiopental anesthesia drug and normal saline solution containing 0.9% sodium were purchased from Exir Pharmaceutical Company, Iran. Other materials were purchased from Sigma-Aldrich (USA).
Animals and chemical treatments: Forty male BALB/c mice (weighing 25-35 g, six weeks’ age) were obtained from the Center of Comparative and Experimental Medicine, Hormozgan University of Medical Sciences. They were housed in a temperature (23±2°C) and air humidity-controlled room (60%). They were maintained on a 12 hr light/dark cycle for ten days before the start of the experiments. The animals were allowed free access to food and water. This study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (IR. HUMS.REC.1398.020).
Mice were randomly divided equally into four groups (n=5). The first group was used as a control (0.5 ml normal saline). Groups 2-4 were treated with NAC [160 mg/kg Body Weight (BW)], deltamethrin (50 mg/kg BW), and NAC plus deltamethrin, respectively 14. The animals were treated for 1 or 24 hr by intraperitoneal (i.p.) injection of compounds 15. At the end of the treatment period, the animals were anesthetized with sodium thiopental (25 mg/kg i.p.), and after the surgery, the blood was obtained directly from liver samples for biochemical and oxidative stress analysis. The serum was isolated from the blood, and Aspartate aminotransferase (AST), Alanine Transaminase (ALT), Alkaline phosphatase (ALP), Lactate dehydrogenase (LDH) levels were measured using the appropriate method explained below. Furthermore, the liver samples were used to measure the ROS and GSH levels.
Measurement of liver/body weight ratio: Liver weight is one of the most critical indicators of liver health, which should be in the range of 3-5% of rat body weight. If the liver is damaged, this ratio will change 16. The liver/body weight ratio was evaluated in the animals of each group after receiving the drug on an electronic weighing scale.
Measurement of AST level: AST level in the blood is used as a biomarker for the diagnosis of liver parenchymal cell damage. In this study, AST was evaluated according to the method of Reitman and Frankel 17.
Measurement of ALT level: ALT level in the blood is used as a biomarker to diagnose liver parenchymal cell damage similar to AST. In this study, ALT was evaluated according to the method of Reitman and Frankel 17.
Measurement of ALP level: ALP level in the blood is used as a biomarker for the diagnosis of liver duct cell damage. In the present study, ALP was evaluated according to the method of Young et al 18.
Measurement of LDH level: LDH level in the blood is used as a biomarker for the diagnosis of liver necrosis. In this study, LDH was evaluated according to the previous method reported by Larsen et al 19.
Measurement of liver tissue ROS level and GSH content: In the present study, the liver tissue ROS level was evaluated according to the pervious method described by Momtaz et al 20. Moreover, GSH content was calculated and normalized according to Tietz's method in each group 21.
Data analysis: All measurements were performed with triplicates. All studies were performed in at least three independent experiments and data are expressed as mean±SD. For comparison between more than two experimental groups, one-way ANOVA following Tukey test was used. Probability p<0.05 were considered statistically significant.
Results :
Evaluation of liver/body weight ratio: This study assayed and compared liver/body weight ratio in the drug-treated mice 1 and 24 hr after receiving the drug in separate groups. The results showed that liver/body weight ratio one hour after receiving medication, showed no significant difference in any group. The liver/body weight ratio was in the normal range of 3-5% of body weight in all groups. Also, the study results showed that the liver/body weight ratio 24 hr after receiving the medication in group receiving deltamethrin alone had a liver/body weight ratio near 5%, showing a significant difference from the control group (p<0.001). The group receiving deltamethrin with NAC, liver/body weight ratio was in the normal range, close to the control group and the group receiving only NAC. It was significantly lower than the group receiving deltamethrin alone (p<0.001). The control group's liver/body weight ratio and the group that received only NAC were also in the normal range (Figure 1).
Evaluation of AST level: In this study, to evaluate the hepatic parenchymal cell damage, we assayed and compared AST enzyme levels in the serum in mice after 1 and 24 hr of receiving the treatments. The results showed that AST level after one hour in the drug group treated with NAC and in the group treated with NAC+deltamethrin was not significantly different from the control group. However, the AST level in the group treated with the deltamethrin was significantly different from the control group (p<0.05). The study results also showed that AST level 24 hr after receiving the drug in the group treated with NAC was not significantly different from the control group. The group treated only with deltamethrin was significantly different in comparison with the control group (p<0.001). However, there was a significant difference in AST levels between the group treated with NAC+deltamethrin and the group treated with deltamethrin alone (p<0.05) (Figure 2).
Evaluation of ALT level: In this study, to evaluate the liver parenchyma cell damage, we assayed and compared ALT enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the drug. The results showed that ALT level one hour after receiving the medication in the group treated with NAC was not significantly different in comparison to the control group. However, ALT level in the group treated with deltamethrin was significantly different from the control group (p<0.001). The group treated with NAC+deltamethrin was significantly different from the group treated with NAC only. The study results also showed that ALT level 24 hr after receiving the medication in the group treated with NAC was significantly different from the control group (p<0.05). However, the ALT level in the group treated with deltamethrin was significantly different from the control group (p<0.001). There was not a significant difference in ALT levels in the group treated with NAC+deltamethrin compared to the group treated with NAC alone (Figure 3).
Evaluation of ALP level: In this study, to evaluate the liver duct cell damage, we assayed and compared ALP enzyme levels in the serum of drug-treated mice 1 and 24 hr after receiving the drug. The results showed that ALP level one hr after receiving medication in the group treated with NAC was not significantly different from the control group. However, the ALP level in the group treated with deltamethrin was significantly different from the control group (p<0.001). The group treated with NAC+ deltamethrin was very different in comparison to the group treated with NAC and the control group (p<0.05). The study results also showed that ALP level 24 hr after receiving the medication in the group treated with NAC was not significantly different from the control group. However, ALP level in the group treated with deltamethrin was significant (p<0.001) only when compared to the control group. The group treated with NAC+deltamethrin was not significantly different from the control group (Figure 4).
Evaluation of LDH level: In this study, to evaluate the liver duct cell damage, we assayed and compared LDH enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the drug. The results showed that LDH level one hr after receiving medication in all three groups (the group treated with NAC, the group treated with deltamethrin, and the group treated with NAC+deltamethrin) was significantly different from the control group (p<0.001). Also, the study results showed that LDH level 24 hr after receiving the medication in all three groups [the group treated with NAC (p<0.001), the group treated with deltamethrin (p<0.001), and the group treated with NAC+deltamethrin (p<0.001)] was significantly different from the control group (Figure 5).
Evaluation of liver GSH content: This study assayed and compared liver GSH content in the drug-treated mice 1 and 24 hr after receiving the drug in separate groups. The results showed that liver GSH content was higher one and 24-hr after receiving medication. There was no significant difference between the control group with groups that received only NAC and NAC with deltamethrin. However, the liver GSH content in the group that received only deltamethrin was significantly lower among the control and other groups (p<0.001) (Figure 6).
Evaluation of liver tissue ROS level: This study assessed liver tissue ROS levels in the drug-treated mice 1 and 24 hr after receiving the drug in separate groups. The results showed that liver tissue ROS level one and 24 hr after receiving medication in the group receiving deltamethrin alone was significantly higher than the control group (p<0.001), and in the group receiving NAC alone there was no change in control group. However, the liver tissue ROS level in the group that received deltamethrin with NAC was higher than the control group (p<0.05) and in the group that received NAC alone (p<0.05) (Figure 7).
Discussion :
In this study we investigated the antidote effects of NAC against deltamethrin-induced hepatotoxicity in mice. The results of this study showed that mice receiving NAC+deltamethrin had more liver GSH content, and lower hepatic enzymes (AST, ALT, ALP) and ROS level than mice that received deltamethrin alone. Also, mice that received NAC+deltamethrin had more liver/body ratio than mice that received deltamethrin alone. This result shows that NAC could be one of the options acting as antidote against deltamethrin poisoning.
The result has shown NAC could increase liver GSH content. Sulaiman et al study conducted in Pakistan in 2020 showed that NAC could significantly increase GSH content in mice receiving acetaminophen 22. Another study which was conducted on murine model in United States in 2017 showed that in the group receiving NAC+acetaminophen had significantly higher GSH content than group that received acetaminophen alone 23. A study was conducted in Saudi Arabia in 2003 and showed that NAC could increase GSH content in rats’ azathioprine induced hepatotoxicity 24. El-Yamany et al study in Egypt in 2021 showed that NAC could significantly increase GSH content in rats’ carbon tetrachloride (CCl4) induced hepatotoxicity for 8 weeks 25. A clinical study was conducted in Hyderabad, India on patient who had anti-tuberculosis drug induced hepatotoxicity. The study demonstrated that NAC could increase liver GSH content and has hepatoprotective effects against anti-tuberculosis drug 26.
Our study had shown NAC could reduce hepatic enzymes as AST, ALT, ALP, LDH against deltamethrin. Avizeh et al’s study in Iran in 2010 was conducted on cats with acetaminophen-induced hepatotoxicity. NAC could significantly reduce hepatic enzymes as AST, ALT, ALP, LDH and protect liver against acetaminophen 27. Another study was conducted in Saudi Arabia in 2003 which showed that NAC could reduce hepatic enzymes such as AST, ALT, ALP, LDH in rats’ azathioprine induced hepatotoxicity 24. Sukumaran et al’s study was conducted in Hyderabad, India on patients who were involved with anti-tuberculosis drug induced hepatotoxicity. The study demonstrated that NAC could reduce hepatic enzymes such as AST, ALT, ALP with hepatoprotective effects against anti-tuberculosis drug 26. Kaya et al’s study was conducted in Turkey in 2023 on adriamycin-induced liver injury in rats. They showed that NAC could significantly reduce hepatic enzymes such as AST, ALT, ALP and protect liver against adriamycin 28.
Our study had shown that NAC could reduce oxidative stress against deltamethrin. A study was conducted in Iran in 2014 on freshly isolated rat hepatocytes induced hepatotoxicity by statins drugs; it showed that NAC could reduce ROS levels significantly and control oxidative stress 29. Sukumaran et al study was conducted in Hyderabad, India on patients who had anti-tuberculosis drug induced hepatotoxicity. They showed that NAC could reduce ROS levels and has hepatoprotective effects against anti-tuberculosis drug 26. Sathish et al’s study which was conducted in India had shown that NAC could reduce oxidative stress with hepatoprotection effects on rats’ dimethylnitrosamine (DMN) induced oxidative stress and hepatotoxicity 30.
Conclusion :
In this experimental study on mice induced hepatotoxicity by deltamethrin, NAC could reduce hepatic enzymes such as AST, ALT, ALP, LDH and oxidative stress and increase liver GSH content. According this result NAC could protect liver against deltamethrin. More studies and clinical trials are needed to prove the results of this study, but NAC could be one of the options as antidote against deltamethrin in deltamethrin poisoning and suicide with deltamethrin.
Ethical approval :
Animals were treated according to the guideline of the Ethics Committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1398.020).
Consent for Publication :
All authors declare consent to publish the article.
Acknowledgement :
The authors of this manuscript wish to express their appreciation to Hormozgan University of Medical Sciences, Bandar Abbas, Iran. This study was approved by the Ethics Committee of Hormozgan University of Medical Sciences (IR.HUMS.REC.1398.020).
Funding: This work was supported by the Research Grants from the Hormozgan University of Medical Sciences, Bandar Abbas, Iran (no; 980079).
Conflict of Interest :
The authors declare no competing interest.
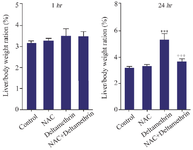
Figure 1. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on liver/body weight ratio (%) of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks *** and +++ represents significantly different p< 0.001 as compared with the control and NAC groups, respectively.
|
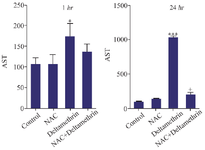
Figure 2. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on AST enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks *, and *** represents significantly different p<0.05, and p<0.001 as compared with the control group, and + represents significantly different p<0.05 as compared with the NAC group, respectively.
|
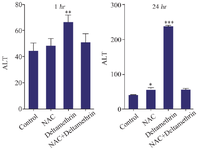
Figure 3. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on ALT enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks *, **, and *** Represents significantly different p<0.05, p<0.01, and p<0.001 as compared with the control group, respectively.
|
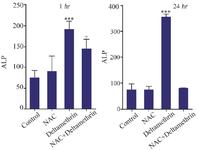
Figure 4. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on ALP enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks ***, and + represents significantly different p<0.001, and p<0.05 as compared with the control and NAC groups, respectively.
|
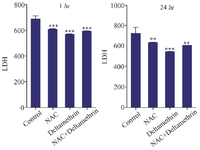
Figure 5. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on LDH enzyme level in the serum of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks **, and *** Represents significantly different p<0.01, and p<0.001 as compared with the control group, respectively.
|
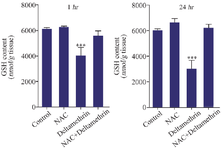
Figure 6. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on liver GSH content of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisk *** Represent significantly different p<0.001 as compared with the control group.
|
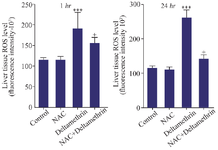
Figure 7. Effect of NAC, Deltamethrin, and NAC+Deltamethrin on liver tissue ROS levels of drug-treated mice 1 and 24 hr after receiving the treatments. Control group received 0.5 ml normal saline 0.9%, intervention groups received NAC (160 mg/kg), Deltamethrin (50 mg/kg), and NAC plus Deltamethrin. Data are given as mean±SD (n=8). Asterisks *** and + represents significantly different p<0.001, and p<0.05 as compared with the control and NAC groups, respectively.
|
|