Gypenosides Production and Spermatogenesis Recovery Potentials of Extracts from Cell Suspension Cultures of Gynostemma pentaphyllum
-
Nguyen-Thanh , Tung
-
Faculty of Basic Science, University of Medicine and Pharmacy, Hue University, Hue 49000, Vietnam
-
Institute of Biomedicine, University of Medicine and Pharmacy, Hue University, Hue 49000, Vietnam
-
Dang-Ngoc , Sang
-
Institute of Biotechnology, Hue University, Hue 49000, Vietnam
-
Vo Nguyen Giap Gifted High School, Quang Binh 47000, Vietnam
-
Tran-Quoc, Dung
-
University of Education, Hue University, Hue 49000, Vietnam
-
 Hoang-Tan, Quang
Institute of Biotechnology, Hue University, Hue 49000, Vietnam, Tel: +84 983735509; E-mail: htquang@hueuni.edu.vn
Hoang-Tan, Quang
Institute of Biotechnology, Hue University, Hue 49000, Vietnam, Tel: +84 983735509; E-mail: htquang@hueuni.edu.vn
Abstract: Background: Gynostemma pentaphyllum (GP), also called Giao-co-lam, is a traditional Vietnamese herb, also known as the "Herb of Immortality", that grows throughout Asian countries and is used for the treatment of hematuria, edema in the pharynx and neck, tumors, and trauma.
Methods: In this study, we investigated the effects of culture conditions on cell growth and total gypenoside accumulation in the GP suspension cells. Cells were cultured on Murashige and Skoog (MS) medium supplemented with 2.0 mg/L KIN and 0.5 mg/L IBA, and different inoculum sizes (2-4 g) for 10-24 days.
Results: The results showed that the optimum inoculum size and shaking speed were 3 g of callus and 120 rpm, respectively. The highest cell biomass reached was 5.833 g of fresh weight, corresponding to 0.136 g of dry weight after 20 days of culture. The total gypenoside and Rb1 accumulation was the highest after 18 days of culture, with 46.498 mg/g and 0.038 mg/g dry weight, respectively. In addition, the crude extract from GP cell suspension cultures remarkably improved pathological changes in mouse testicular tissue after scrotal heat exposure. Blood testosterone levels were significantly increased in heat-exposed mice treated with the GP cell suspension culture extract.
Conclusion: Taken together, these results suggest that GP bio-mass production by cell suspension cultures leads to the accumulation of gypenosides in large amounts, and provides the potential for the recovery of spermatogenesis following heat stress.
Introduction :
Gynostemma pentaphyllum (Thunb.) Makino (GP) belongs to the Gynostemma, Cucurbitaceae family, and is a perennial creeping herb 1. GP is a medicinal plant with potential pharmacological activities such as antioxidant, antitumor, immunopotentiating, cholesterol-lowering, hypoglycemic, and antidiabetic effects 2. More than 180 gypenosides, flavonoids, and polysaccharides have been isolated and characterized from GP 3. In Vietnam, GP has been used as a folk medicine to treat cough and chronic bronchitis and is distributed from plains to mountainous areas at altitudes of up to 2000 m 4.
Gypenosides, dammarane saponins isolated from GP, are the most important bioactive compounds that respond to their biological activities and clinical effects 4,5. The chemical structure of gypenosides closely resembles that of the ginsenosides in Panax ginseng 2. The eight saponins found in GP are similar to the protopanaxadiol-type in Panax ginseng, including ginsenosides Rb1 (Gypenoside III), Rc, Rb3, Rd (Gypenoside VIII), F2, Rg3, malonyl-Rb1, and malonyl-Rd 5.
Numerous bioactive compounds are produced in plants through the application of bioreactors to cultivate plant cells and organs. Plant cell culture in a bioreactor has many advantages over classical tissue culture such as easy optimization of the real-time temperature, nutrients, oxygen concentration, and pH of the medium. On the other hand, the cost and time consumed are reduced, the quality of products is enhanced without pesticide contamination, and production is independent of cultivation season or geographical constraints 6. However, there is limited information on gypenoside production in GP cell culture 2. However, in vitro gypenoside production has been reported, such as in hairy root cultures 2 or calli 7. Gypenoside production in cell suspension cultures has not been well studied.
GP extract has been reported to affect cell and animal models of several diseases such as chronic liver disease 8, dermal cell aging 9, obesity 10, and cancer 11. GP has been proposed to correlate with multiple signaling pathways, including apoptosis, cell cycle arrest, inhibition of glycolysis, immunomodulatory activity, and antioxidant activity by upregulating SIRT1 and regulating the EGFR/PI3K/Akt signaling pathway 10,12. Furthermore, several clinical studies have shown that the GP extract could have potent curative effects on cancer 11, chronic stressful conditions 13, treating obese individuals 14, and improving body composition in overweight males and females 15.
This study aimed to establish a suspension cell culture system of Gynostemma pentaphyllum for gypenoside production, analyze the gypenosides accumulated in the cells, and investigate the effect of suspension cell biomass extract on spermatogenesis in mice.
Materials and Methods :
Cell suspension culture: GP calli were provided by the Laboratory of Gene Technology, Institute of Biotechnology, Hue University, Vietnam 16. Cell suspension culture was conducted by transferring 2 g of 30-day-old calli into a 250 ml Erlenmeyer flask containing 50 ml of Murashige and Skoog (MS) liquid medium supplemented with 2.0 mg/L KIN and 0.5 mg/L IBA. The culture flasks were then incubated at 25±2°C, shaking at 120 rpm 17. Suspension cells were harvested every two days, starting from day 10 to day 24. The fresh cell biomass was filtered, washed with distilled water, and weighed. Dry weight was determined by drying the fresh biomass at 50°C until a constant weight was achieved. Cell growth and gypenoside accumulation during the culture period were estimated.
To investigate the effects of inoculum size on gypenoside accumulation, culture systems with different inoculum sizes (2-4 g of calli per flack) were used. Suspensions of cells were collected on the day with the highest total gypenoside concentration, as previously determined.
Quantification of gypenosides accumulation: Dried suspension cells of GP (0.5 g) were extracted with 5 ml of 80% methanol, ultrasonicated for 30 min, repeated three times, and evaporated at room temperature. The residues were resuspended in methanol and filtered through a 0.22 μm membrane. The gypenoside solution was maintained at 4°C for further experiments 18.
Total gypenosides quantification: The total gypenoside concentration was estimated using colorimetric methods 19,20. Ten mg of gypenosides residue were dissolved in 5 ml of 80% methanol. Fifty µl of the solution was transferred to another test tube and 0.25 ml of vanillin reagent (8% w/v) was added. The reaction solution was placed on ice and 2.5 ml of 72% (v/v) sulfuric acid was added, mixed, and left for 3 min. The test tubes were warmed to 60°C for 10 min and then cooled on ice. Absorbance was measured at 544 nm using a UV-Vis scanning spectrophotometer (Spectro UV-2650, Labomed, Inc.). The ginsenoside content was calculated based on ginsenoside Rb1 (Y0001347, CRS) standard curves.
Rb1 quantification: The residue was resuspended in methanol to a final concentration of approximately 5 mg/ml and filtered (0.22 μm). Rb1 content was determined by HPLC using an Alliance E2695 system with a C18 column (4.6 mm ×250 mm×5 mm). The mobile phase consisted of water (66%) and acetonitrile (34%) for 20 min, a column temperature of 30°C, a flow rate of 0.8 ml/min, and an injected volume of 20 µl. The signal was measured using a PDA2998 detector at a wavelength of 203 nm 21. The Rb1 content was calculated based on the peak area of the HPLC dendrogram compared to the standard Rb1.
Animal model for heat stress-induced spermatogenesis: The animal experimental protocol was reviewed and approved by the Hue University of Medicine and Pharmacy (certificate no. H2019/345). Adult male Swiss mice, aged 8-10 weeks old (20-23 g), were purchased from the Pasteur Institute of Nha Trang, Vietnam. Mice were kept in an animal facility at a controlled temperature (25±1°C) and photoperiod (12 hr light: 12 hr dark) with free access to food and water. The animals were randomly divided into four groups of 10 mice each: Control group (CON), 40°C Heat exposure group (H40), 40°C heat exposure and treatment with extraction of natural products from GP at 200 mg/kg group (H40+N), 40°C heat exposure and treatment with extraction of biomass products from GP cell suspension culture at 200 mg/kg group (H40+CS).
The heat-stress procedure was performed as described in a previous study. In brief, the lower half of the mouse's body, including the scrotum, was submerged in a temperature-controlled water bath at 40°C for 10 min, twice per day, at 10 min intervals, 6 days per week for 5 weeks. Control mice were treated in the same way but in a water bath maintained at 25°C. After the bath, the mice were dried and examined for any injury or redness of the scrotal skin before being returned to their cages. Mice in the four groups were housed in different cages and had free access to water and food. All animals were cared for under identical environmental conditions. Mice were monitored for general health. After completing the heat exposure experiment for five weeks, all mice were sacrificed under anesthesia. Blood and testicular tissues were harvested for testosterone and histological morphometric analyses.
Tissue processing and hematoxylin-eosin staining: At the end of the surgical procedure, testicular specimens were individually immersed in 4% buffered formaldehyde, dehydrated with graded concentrations of ethyl alcohol, and embedded into paraffin blocks. Paraffin sections 5 μm thick were obtained and transferred onto gelatin-coated slides, deparaffinized with xylene, and then rehydrated through a descending series of ethanol and water. The slides were stained with Hematoxylin and Eosin (H&E) and observed under a light microscope for histopathological analysis.
Blood sampling and plasma testosterone assays: Blood samples were harvested from anesthetized mice by heart puncture using a syringe and collected in EDTA blood collection tubes. Serum was obtained after centrifugation at 2000 rpm at 4°C for 20 min. Plasma testosterone concentration was measured by electrochemiluminescence immunoassay using Elecsys Testosterone II (05200067190; Roche, Basel, Switzerland), according to the manufacturer’s instructions.
Statistical analysis: All cell culture and gypenoside quantification experiments were repeated thrice. Means were compared using one-way analysis of variance (ANOVA), followed by Duncan’s test (p<0.05).
Results :
Biomass production by suspension cell cultures: A preliminary investigation of the growth of GP suspension cells showed that the growth rate was low during 0-10 days of culture, in which fresh biomass increased from 2.000 to 2.280 g/flask (data not shown). Thus, cell biomass and gypenoside content were estimated only after 10 days of incubation. As shown in figure 1, the cell biomass increased continuously from 10 to 20 days of culture. The highest fresh biomass reached was 3.780 g/flask (0.087 g dry biomass). Cell biomass accumulation dramatically decreased, accumulating only 3.010 g and 0.059 g of fresh and dry weight, respectively, after 24 days of culture.
Kinetic gypenosides accumulation in cell suspension culture: The accumulation of Rb1 and gypenosides in suspension cells reached the highest value 2-4 days earlier than biomass accumulation (Table 1). The total gypenosides increased from day 10 to day 18, reaching a maximum of 42.122 mg/g dry weight, and then decreased after 20 days of cultivation. There were no significant differences in gypenoside concentration between days 16 and 18. The biosynthesis of Rb1 was not relevant to gypenoside accumulation. Gypenoside content in cells increased from 10 to 18 days to a maximum of 42.122 mg/g dry weight. Meanwhile, Rb1 content was maintained in the cell until day 20, and then decreased sharply from days 22 to 24.
Optimization of culture conditions for gypenosides biosynthesis: Table 2 shows the data on the growth and gypenoside accumulation of 16-20-day suspension cell lines that differed in inoculum sizes (2-4 g callus). The results indicated that the fresh biomass reached the highest value on day 20 when the inoculum sizes were 2 and 3 g. Meanwhile, the biomass reached its highest value earlier with 4 g inoculum. Increasing the inoculum size to 3 g and 4 g also enhanced biomass accumulation in comparison with an inoculum size of 2 g, reaching 5.833 g, 5.430 g, and 3.781 g, respectively. The data suggest that cell growth may require a certain initial density of cells, where lower or higher inoculum sizes inhibit the growth of the suspension cell culture. HPLC analysis of gypenoside showed the highest concentration of 46.498 mg/g dry weight after 18 days of culture with an inoculum size of 3 g. In contrast, the highest Rb1 concentration was observed in the culture system with 2 g of inoculum. The present study suggests that the inoculum size of the GP suspension cell culture may vary depending on the research purpose where gypenodises or Rb1 production is targeted.
Cell biomass of GP cell recovery of spermatogenesis after heat stress: Control mice without heat exposure and those who didn't receive GP extract showed histopathology of normal testicles with the complete process of spermatogenesis in which progenitor spermatogonia developed into mature spermatozoa in the seminiferous tubules (Figure 2A). Meanwhile, chronic heat exposure at 40°C for 5 weeks (H40 group) impaired the histological architecture of the testicular tissue, and spermatogenesis was completely arrested. The testicular histology of the heat-exposed group exhibited degenerated and disorganized features of spermatogenic epithelium and reduced spermatogenic cell numbers (Figure 2B). Heat-exposed mice receiving GP extract from natural plants (H40+N group) or cell suspension cultures (H40+CS group) showed histological recovery of testicular tissue compared to those in the heat-stress exposure group. Several round and elongated spermatids were found in the spermatogenic epithelium near the lumen. Spermatogenesis was ameliorated with the differentiation process that occurs in the seminiferous epithelium observed in the testis of heat-exposure mice receiving GP extract (Figures 2C and D).
Plasma testosterone assay was performed to assess spermatogenesis. Figure 2E shows that GP extract attenuated the heat stress-induced disruption of spermatogenesis. Control mice had higher levels of plasma testosterone than those exposed to heat. Chronic heat exposure at 40°C for five weeks significantly suppressed serum testosterone levels. Heat exposure may disrupt testosterone biosynthesis in mice. Interestingly, GP extract from natural plants and cell suspension culture significantly increased plasma testosterone levels compared to those in mice that received only heat stress. There was no significant difference in testosterone levels in mice that received the GP extract from natural plants or cell suspension cultures.
Discussion :
Plant cell suspension culture in a bioreactor, an incessant, sustainable, economical, and viable pro-duction of secondary metabolites, has been considered a powerful tool for the production of bioactive compounds from medicinal plants 22. Numerous compounds in medicinal plants are produced through plant tissue culture, such as catechin from Ajuga bracteosa 23 and eurycomanone from Eurycoma longifolia 24. Production of high-value active secondary metabolites at the industry al level, such as shikonin, berberine, and sanguinarine, has been achieved in cell cultures of Lithospermum erythrorhizon, Coptis japonica, and Papaver somniferum, respectively 25.
Only a few studies have been conducted on the accumulation of bioactive metabolites in in vitro cultures of Gynostemma pentaphyllum (G. pentaphyllum). For example, Chang et al established a hairy root culture system of G. pentaphyllum and found that the dry biomass of hairy roots grown in MS medium for 49 days was 7.3 g/L with a gypenoside content of 38 mg/g dry weight 2. Gypenoside is also produced from the callus culture of G. pentaphyllum 7,16. Gypenoside production has not been well studied, except for the first results of our studies 26,27.
Our data confirmed that both gypenosides and Rb1 accumulated the most after 18 days of suspension cell culture. Thus, further gypenoside and Rb1 accumulation studies were conducted on suspension cells harvested after 18 days of culture. Moreover, gypenosides in a cell line are higher than those of callus and stem 16, and similar to a hairy root in a previous report 2.
In particular, inoculum size had a positive effect on biomass and metabolite production up to an optimum concentration 28. In this study, the optimal inoculum size for biomass accumulation and gypenoside production was 3 g of callus per 50 ml culture medium (6% w/v), which is similar to that reported by Loc et al for the production of madecassoside in Centella asiatica (L.) suspension cell culture 29. Nhan et al also found that an inoculum size of 6% (w/v) is optimal for eurycomanone biosynthesis in long jack (Eurycoma longifolia) suspension cells culture 24. In other reports, the optimal initial inoculum sizes for secondary metabolic compound production were lower, such as 2% for flavonoid production from Ficus deltoidea var. kunstleri cell 17, or 3.2% in anthocyanin production from Vitis vinifera cell 30.
Our histological examination and plasma testosterone analysis indicated that both GP extract from natural plants and cell suspension culture attenuated heat stress-induced damage to testicular tissue and spermatogenesis complications. For centuries, many herbs have been used by humans in folk medicine as therapeutics for male reproductive impairment, such as bioactive compounds (Curcumin, Ellagic Acid, Vitamin C, Vitamin E) and natural products with bioactive compounds (Garlic, Ginger, Grape, Green Tea) 31. Heat stress can negatively affect male reproduction in mammals. Arun et al showed that Phyllanthus emblica leaf extract protects against testicular damage in rats with chronic stress by changing to functional proteins, especially testicular tyrosine-phosphorylated proteins 32. Green tea extract (Camellia sinensis) has been reported to lead to better recovery of sperm motility and morphology after testicular heat shock in rats 33. Hou et al show that twenty-five herbs in the Duzhong Butiansu prescription improved heat stress-induced spermatogenic disorders by regulating sperm formation and the HSF/Hsp70 signaling pathway 34.
Conclusion :
In conclusion, the highest gypenoside concentration in suspension cells obtained in the 50 ml MS liquid medium culture system contained 3 g of inoculum size and a shaking speed of 120 rpm. The total gypenosides and Rb1 accumulation reach their highest after 18 days of culture with 46.498 mg/g and 0.038 mg/g dry weight, respectively. This study provides evidence for the efficient use of GP suspension cells to accelerate the production of gypenosides and Rb1. GP cell suspension biomass extract attenuates heat stress-induced disruption of spermatogenesis in seminiferous tubules and plasma testosterone level.
Acknowledgement :
This work was partially supported by Hue University under the Core Research Program Grant No. NCM.DHH2020.12. We thank the reviewer and approval by the Hue University of Medicine and pharmacy ethics committee.
Conflict of Interest :
No conflict of interest.
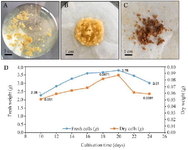
Figure 1. Cell growth kinetics and biomass accumulation of Gynostemma pentaphyllum using cell suspension culture.
- A) Gynostemma pentaphyllum suspension cell culture, B) Fresh biomass accumulation, C) and dried biomass, D) Growth kinetics curves of fresh and dry weights of suspension cells in MS liquid medium supplemented with 2.0 mg/L KIN and 0.5 mg/l IBA on culturing days.
|
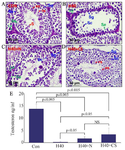
Figure 2. Attenuation of heat-stress-induced spermatogenesis complications by GP extract in mice. A) Normal spermatogenesis in the seminiferous tubules in the testes of the control group (CON). B) Spermatogenesis in seminiferous tubules disruption in heat stress-induced mice at 40°C (H40). C) Extraction of natural products from plant recovery spermatogenesis (H40+N). D) Extraction of biomass products from cell suspension culture recovered spermatogenesis (H40+CS). E) The extraction of natural products from plants and biomass products from cell suspension cultures recovers blood testo-sterone levels. Le: Leydig cells, Se: Sertoli cells, Sg: Spermatogonia, Sc: Spermatocytes, rs: round spermatids, es: elongating spermatids, S: Spermatozoa
|
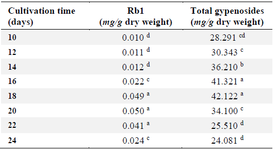
Table 1. Gypenosides accumulation in cell suspension culture
Different letters (a, b, c, …) in each column indicate significantly different means (Duncan’s test, p<0.05).
|
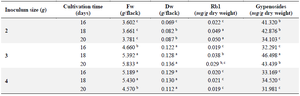
Table 2. Effects of inoculum sizes on cells growth and gypenosides accumulation
Different letters (a, b, c, …) in each column indicate significantly different means (Duncan’s test, p<0.05).
|
|