Human T2R38 Bitter Taste Receptor Expression and COVID-19: From Immunity to Prognosis
-
Baghsheikhi , Hediyeh
-
Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
USERN Office, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Soltani , Afsaneh
-
Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
USERN Office, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Mafi , Zahedeh
-
Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
USERN Office, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
Samieefar, Noosha
-
Student Research Committee, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
USERN Office, Shahid Beheshti University of Medical Sciences, Tehran, Iran
-
 Khazeei Tabari , Mohammad Amin
Mazandaran University of Medical Sciences, Sari, Iran, Tel: +98 9118228029; E-mail: Aminkhazeeitabari@gmail.com
Khazeei Tabari , Mohammad Amin
Mazandaran University of Medical Sciences, Sari, Iran, Tel: +98 9118228029; E-mail: Aminkhazeeitabari@gmail.com
-
USERN Office, Mazandaran University of Medical Sciences, Sari, Iran
Abstract: Background: Bitter taste-sensing type 2 receptor (T2Rs or TAS2Rs) found on ciliated epithelial cells and solitary chemosensory cells have a role in respiratory tract immunity. T2Rs have shown protection against SARS-CoV-2 by enhancing the innate immune response. The purpose of this review is to outline the current sphere of knowledge regarding this association.
Methods: A narrative review of the literature was done by searching (T2R38 OR bitter taste receptor) AND (COVID-19 OR SARS-CoV-2) keywords in PubMed and google scholar.
Results: T2R38, an isoform of T2Rs encoded by the TAS2R38 gene, may have a potential association between phenotypic expression of T2R38 and prognosis of COVID-19. Current studies suggest that due to different genotypes and widespread distributions of T2Rs within the respiratory tract and their role in innate immunity, treatment protocols for COVID-19 and other respiratory diseases may change accordingly. Based on the phenotypic expression of T2R38, it varies in innate immunity and host response to respiratory infection, systemic symptoms and hospitalization.
Conclusion: This review reveals that patients’ innate immune response to SARS-COV-2 could be influenced by T2R38 receptor allelic variations.
Introduction :
The innate immune system is crucial in fighting against Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2), as is the case with most infections 1. Inhaled pathogens and particles naturally pose a constant threat to the respiratory system. Mucociliary Clearance (MCC), which consists of two components, including mucus production and mucus transport, is the primary physical defense against such irritants 2-4. Debris-laden mucus is transported to the oropharynx by ciliary beating and removed by swallowing or expectoration 2,5. Various environmental and host stimuli increase ciliary beating rate via various secondary messenger routes, such as intracellular Ca+2 and Nitric Oxide (NO) production 2,6-9. The wider literature suggests bitter taste-sensing type 2 receptor (T2Rs or TAS2Rs), found on ciliated epithelial cells and solitary chemosensory cells, have a function in sinonasal immunity 2,10,11. T2R38 is one of the numerous T2R isoforms found in human motile cilia 1,2. When T2R38 is stimulated by Propylthiouracil and phenylcarbamide, NO is produced and antimicrobial peptides are released 12. NO increases MCC and kills pathogens in the human respiratory tract mucosa 12,13. Two common haplotypes result from three single-nucleotide variants in the gene which encodes T2R38, including the functional variant Proline-Alanine-Valine (PAV) and the nonfunctional variant Alanine-Valine-Isoleucine (AVI) 1,14,15. When compared to those homozygous for nonfunctional T2R38 (AVI/AVI) and heterozygous for this receptor (PAV/AVI), individuals with functional T2R38 (PAV/PAV), had fewer gram-negative upper respiratory infections and a better quality of life 16,17. Several studies have investigated the association between T2R38 phenotype expression and COVID-19 severity and prognosis 1,16,18. The purpose of this review is to outline the current sphere of knowledge regarding this association.
Materials and Methods :
This study was a narrative review of literature which was done by searching (T2R38 OR bitter taste receptor) AND (COVID-19 OR SARS-CoV-2) keywords. Electronic databases including PubMed and Google scholar was searched to find articles investigating the association of human T2R38 bitter taste receptor expression and COVID-19.
Results :
What is T2R38?
The role of bitter taste is protective against toxin ingestion. The bitter taste sense arise from G-Protein Coupled Receptors (GPCRs) found in type II taste receptor cells in the oral taste buds T2Rs, present on extra-oral ciliated epithelial cells and solitary chemosensory cells (first line defence elements in tracheobronchial pathway) that have been linked to sinonasal innate immunity 19-21. T2Rs are GPCRs which take on various isoforms. There are 25 known T2Rs in the human body. T2R38 is a type of T2Rs that responds to chemicals with thiourea (N-C=S). Furthermore, these receptors can respond to other compounds containing (N=C=S). T2R38 is also one such isoform populating motile cilia in human mucosal linings, including airways, human placenta, and colon 21. T2R38 is encoded by the TAS2R38 gene in the human genome expressed as two types of haplotypes, the functional variant PAV and the non-functional variant AVI.
Role of T2R38 in innate immunity: T2Rs have little influence on taste perception within the extra-oral airway. When T2Rs bind to their specific agonists, they respond to a plethora of bitter chemicals, including phenylthiocarbamide (PTC), denatonium benzoate, strychnine, quinine and caffeine 22,23. Ciliated sinonasal epithelial cells are essential for the upper respiratory tract immunity's initial line of defense. To maintain a healthy sinonasal tract, effective MCC necessitates the coordinated ciliary-driven movement of airway surface fluids, which contains mucus-trapped pathogenic organisms and detritus. Stasis of sinonasal secretions and resulting local inflammation occur when MCC is hindered propagating a susceptibility to infection 4,24-27.
Calcium-driven NO generation is a T2R38 innate immunological response. This calcium and NO signaling incorporates two classical components of the taste signaling cascade using a phospholipase C isoform (PLC2) 28,29, and the TRPM5 ion channel in type II taste cells. NO damages the intracellular components of infectious bacteria, and raises ciliary beat frequency by acting on protein kinase G and guanylyl cyclase, resulting in increased MCC activity 29,30. The clearance of mucus-trapped pathogens and dispersion of antimicrobial chemicals in response to pathogens is accelerated by ciliary beat frequency 30,31. Solitary chemosensory cells are nonciliated epithelial cells which express both sweet (T1R2/3) and bitter (T2R) receptors. Although stimulation of T2Rs on ciliated epithelial cells causes a Ca2+-dependent NO response. T2R stimulation on solitary chemosensory cells results in Ca2+ propagation throughout gap junctions into ciliated cells. This releases antimicrobial compounds such as defensins 1 and 2, lactoferrin and others 20,32. Current literature explores oral taste sensitivity to measure extra-oral taste receptor function, where basic taste test may show changes in sinonasal immune functions 32,33. The role of T2R38 receptor against pathogens is illustrated in figure 1.
Role of T2R38 in respiratory infections and diseases: Quorum Sensing (QS) is a mechanism by which Pseudomonas aeruginosa (P. aeruginosa) uses signal molecules to regulate gene expression according to population density. Molecules are sensed by the ciliary airway T2R38 receptors, prompting changes in NO production and ciliary beat frequency, possibly leading to enhanced bacterial clearance 2. Castaldo et al 34 demonstrated frequency of PAV allele was significantly reduced in Cystic Fibrosis (CF) patients with nasal polyps requiring surgery and also in CF patients with chronic pulmonary P. aeruginosa colonization. This confirms the relationship between the altered function of the T2R38 receptor and the risk of lower and upper airway infections and chronic Rhino-Sinusitis (CRS). AVI/AVI genotype is also an independent risk factor for patients with medically recalcitrant CRS 2,35. Adappa et al demonstrated that patients with failing medical treatment for CRS undergoing Functional Endoscopic Sinus Surgery (FESS) revealed frequency of AVI/AVI genotype which was higher in this cohort than in the control population. They also compared the distribution of age, sex, aspirin sensitivity, diabetes, asthma, allergies, nasal polyposis and smoking status in CRS patients requiring FESS. Here, PAV/PAV was over-represented in CRS patients with allergies, asthma, aspirin sensitivity, nasal polyposis and diabetes. Evidence from mouse and human studies suggests that TAS2Rs expressed on airway smooth muscles promote muscle relaxation and bronchodilation, and also regulates migration of white blood cells especially eosinophils 35-37. TAS2R has the potential to be considered as a therapeutic target in obstructive lung disease. Further research is necessary to determine which particular TAS2R receptors are of importance 36. There is paucity within the literature showing correlation between T2R38 genotype and chronic lung conditions.
Role of T2R38 in coronaviruses and COVID-19: T2R38 is activated by phenylthiocarbamide and propylthiouracil 38. NO is generated when T2R38 is stimulated by agonists, which promotes MCC, and destroys pathogenic material in the human respiratory mucosa 10. Åkerström et al 13 discovered that NO suppresses SARS-CoV replication by two separate pathways. NO and its derivatives reduce the palmitoylation of newly generated spike proteins, affecting its fusion with its cognate receptor, ACE-2. The cellular entry receptor for SARS-CoV-2 is Angiotensin-Converting Enzyme 2 (ACE-2), is widely present in the human lower respiratory tract, as well as other tissues 39. NO and its metabolites diminish viral RNA production during the initial phases of viral replication explained by the cysteine proteases encoded in Orf1a gene of SARS-CoV genome sequence. Phenotypic perception of phenylthiouracil bitter sensitivity throughout the course of a person's life has been assessed, and found that sensitivity to the T2R38 agonist, phenylthiouracil, decreases as they get older 40,41. This phenomenon appears to be more common in heterozygotes. Davies et al 42 estimated that susceptibility to COVID-19 infection in individuals younger than 20 years is approximately half that of adult people more than 20 years of age. Those symptomatic accounted for 21% of those aged 10 to 19 years, and 69% in those aged 70 years and older. This used an age-structured mathematical formulation of data from 6 countries illustrating T2R phenotypic expression declines with ageing. A retrospective, cross-sectional study evaluating 100 adult patients positive for SARS-CoV showed that all 21 patients (100%) requiring hospital admission were classified as non-tasters (AVI/AVI), whereas 79 non-hospitalised patients with mild to moderate symptoms were classified as tasters (PAV/AVI) and no supertasters (PAV/PAV). This may necessitate a potential association between outcome and clinical course of COVID-19 and phenotypic expression of T2R38 in relation to hospitalization 16.
Discussion :
In the extra-oral airway, there are bitter taste receptors on various cells with T2Rs present in the epithelial ciliated cells, which are involved in innate immunity when attached to their agonist. Activation of calcium-based T2R38 produces NO that plays three vital roles through its biocidal function:
1. it damages the components inside the microbial cells, 2. increases Ciliary Beat Frequency (CBF), and 3. increases MCC 43-46. It has been proposed that evaluating treatment regimens for COVID-19 patients and other respiratory tract infections in accordance with their TAS2R38 alleles may lead to a beneficial course of action 18. Akerstrom et al 47 showed that NO and its derivatives could reduce the initial production of viral RNA by inhibiting spike protein. Many antibiotics, including macrolides, affect the host’s innate immune response 48,49. One of these off-target effects of bitter-tasting antibiotics, notably Azithromycin, activates T2Rs 50,51. Taste status when using Azithromycin in COVID-19 management has not been assessed profoundly. Quinine derivatives 52 are known agonists of T2Rs, however their effectiveness against COVID-19 has not been thoroughly examined. The effects of Azithromycin were associated with the taste status (measured by the T2R38 phenotype), but Azithromycin was not recognized as a T2R38 agonist 53. Jaggupilli et al 54, showed the highest bitterness score recorded via E-tongue activates Taste 2 Receptor Member 4 (T2R4).
In another research evaluating drug responses based on T2R38, Caly et al purported that Ivermectin reduced the viral load up to 5,000-fold in culture within 48 hours 55. However, the mechanism of Ivermectin against COVID-19 remained unknown. Patients display a variety of clinical features of COVID-19 depending on their different genetic structures. Taha et al demonstrated there is a clear correlation between the T2R38 phenotype and severe acute respiratory syndrome coronavirus (SARS-CoV-2) symptoms duration and severity 1,16. Supertasters with high levels of T2R38 expression showed localized symptoms in the upper respiratory tract. Tasters with moderate levels of T2R38 displayed local and some mild to moderate generalized symptoms such as mild fever. In contrast, non-taster people, who did not express T2R38 alleles, showed more severe infections with generalized symptoms. Intrinsic immunity, in the form of T2R38, acts as a shield against upper respiratory tract pathogens 53. Supertasters include 25% of the population and account for the majority of asymptomatic carriers. Non-taster people account for 25% of the population and usually have severe symptoms. 50% of the population are tasters and can often recover from COVID-19 infection 1. Recent studies have demonstrated that possessing T2R38 alleles (taster) may operate as a protective factor against SARS-CoV-2 infection, while non-tasters may represent a risk factor. According to Risso et al's research 56 that investigated the frequency of tasters and non-tasters in 54 COVID-19 patients in comparison to healthy individuals, a direct correlation between T2R38 phenotypes and vulnerability to show mild or severe symptoms of SARS-CoV-2 infection based on T2R38 allele presentation could not be found. A summary of recent studies regarding association of T3R38 receptor and COVID-19 is available in table 1.
Conclusion :
This review reveals that patients’ innate immune response to SARS-COV-2 was influenced by T2R38 receptor allelic variations. The clinical outcome of patients with SARS-COV-2 infection was correlated with the T2R phenotype. To lessen the severe clinical symptoms linked to the deteriorating respiratory system, cytokine storm, hyper-reactive immune responses, and renal and cardiovascular issues on the SARS-COV-2 patients, prospective bitter agonists of natural origin or derived bio-active should be targeted. This study suggests that COVID-19 treatment protocols may be modified based on T2R phenotypic expression because T2Rs are widely distributed in the respiratory tract and a variety of treatment protocols currently available around the world have yielded conflicting results regarding their efficacy. However, sufficient clinical trials must be conducted to demonstrate the effectiveness of targeting the bitter taste receptors to control the SARS-COV-2 outbreak and its associated pathogenicity on the worldwide population. Additionally, the wide diversity of the T2SR38 gene polymorphism and its potential link to mortality raises questions about the effectiveness of vaccine development programs among various racial groups.
Conflict of Interest :
The authors declare that there is no conflict of interest.
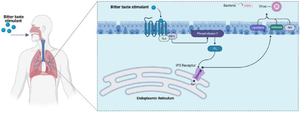
Figure 1. The role of T2R38 receptor against pathogens.
|

Table 1. Summary of studies about the role of T2R38 receptor in COVID-19
|
|