Cell Surface Vimentin Detection in Cancer Cells by Peptide-Based Monoclonal Antibody
-
Sadeghi, Niloufar
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Fazli, Ghazaleh
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Bayat , Ali Ahmad
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Fatemi , Raminasadat
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Ebrahimnejhad , Nasim
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Salimi, Ali
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Zarei, Omid
Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran , Tel: +98 87 33664643; E-mail: omidzarei58@gmail.com; o.zarei@muk.ac.ir
Zarei, Omid
Cellular and Molecular Research Center, Research Institute for Health Development, Kurdistan University of Medical Sciences, Sanandaj, Iran , Tel: +98 87 33664643; E-mail: omidzarei58@gmail.com; o.zarei@muk.ac.ir
-
 Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: 21 22432020; Fax: +98 21 22432021;hodrab@gmail.com; rabbani@ari.ir
Rabbani, Hodjattallah
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel: 21 22432020; Fax: +98 21 22432021;hodrab@gmail.com; rabbani@ari.ir
Abstract: Background: Vimentin is a prominent Intermediate Filaments (IFs) protein expressed in different mesenchymal origin cell types. Besides a wide range of cellular function roles associated with vimentin expression, its dysregulation and cell surface expression in the induction of malignancy properties have been reported extensively, making it a promising cancer-specific target. Therefore, this study aimed to generate and characterize anti-vimentin monoclonal antibodies.
Methods: A 14-mer synthetic peptide from vimentin was conjugated to Keyhole Limpet Hemocyanin (KLH) and used for immunization of Blab/C mice and monoclonal production by conventional hybridoma technology. The monoclonal antibody was purified using affinity chromatography of supernatants from the selected hybridoma cells. ELISA, Immunoprecipitation-Western blotting (IP-WB), Immunocytochemistry (ICC), and flow cytometry were employed to characterize the produced monoclonal antibody in terms of interaction with vimentin immunizing peptide as well as vimentin protein.
Results: Amid the several obtained producing anti-vimentin antibody hybridomas, the 7C11-D9 clone (IgG1 isotype with kappa light chain) showed higher reactivity with the immunizing peptide, and led to its selection for purification and characterization. The purified antibody could detect vimentin protein in IP-WB, ICC and flow cytometry of the normal and cancerous cells with different origin. No vimentin expression was found in normal healthy Peripheral Blood Mononuclear Cell (PBMC).
Conclusion: Taken together, 7C11-D9 anti-vimentin monoclonal antibody might be used as immune diagnostic or immune therapeutic tool where detection or targeting of vimentin in a wide range of organisms is required.
Introduction :
Intermediate Filaments (IFs)- in combination with microfilaments and microtubules- are constructive for the filamentous cytoskeleton system 1. IFs containing different proteins with shared properties in sequence/ structure point of view are classified into six types, nominated as type I to type VI 2. Vimentin is a significant type III protein of IFs composed of 466 amino acids (~53 kDa molecular weight). It is expressed in different mesenchymal origin cell types with expression patterns from nucleus to cell surface 3,4. Although, vimentin is known as a cytoskeleton protein responsible for the maintenance of cell structural integrity, a wide range of cellular functions such as cell adhesion, motility, migration, survival, and wound healing have been associated with vimentin expression and its interaction with other proteins 5-8.
Besides the physiological roles, dysregulation of vimentin has extensively been documented in malignant transformation of breast cancer 9, lung cancer progression 10 and colon cancer metastasis 11. Additionally, several reports have introduced vimentin as a marker for tumor-initiating, circulating tumor cells 12,13 and an indicator for the poor prognosis in cancer patients 14,15. Therefore, there is adequate satisfying evidence showing the drug-ability of vimentin for development of anti-cancer intervention strategies 16-18. Cell-Surface Vimentin (CSV) expression in cancer cells makes it a promising cancer-specific target for developing cell surface targeting agents such as monoclonal antibodies 19,20.
During the recent decades, monoclonal antibodies have gained more attention to be valuable tools in cancer diagnosis, prognosis, and therapy owing to their specific properties to attaching the cell surface components. Consequently, the development of specific monoclonal antibodies against cell surface cancer markers is appreciated for improving the available knowledge in cancer therapy and diagnosis research 21. Based on the above mentions, this study aimed to generate and characterize anti-vimentin monoclonal antibody against a synthetic peptide derived from vimentin protein.
Materials and Methods :
Immunogen peptide designing and conjugation to a carrier protein: First of all, a 14-mer peptide derived from human vimentin (NP_003371.2), from amino acid 347 to 360 (MEENFAVEAANYQD), was selected as an immunogen peptide. A cysteine residue was added to the C-terminal end of the peptide to facilitate its conjugation to the carrier proteins.
The immunograde peptide was ordered for synthesis by Thermo Scientific (Thermo Scientific, USA). It was conjugated to carrier proteins Keyhole Limpet Hemocyanin (KLH) and Bovine Serum Albumin (BSA) separately (Sigma, USA) under identical conditions as described 22. Briefly, 30 ul of DMSO was added to 5 mg of peptide with subsequent dropwise adding 1× Phosphate-Buffered Saline (PBS) to obtain a 5 mg/ml concentration. The peptide-BSA was used for conjugation assessment by its running on a 10% SDS-PAGE gel 23, and employed for performing the immunoassays. The peptide–KLH was subjected to mice immunization for antibody production.
Mice immunization and hybridoma production: Four female BALB/c mice at 8-weeks old were considered for immunization. Briefly, each mouse was intramuscularly injected with peptide-KLH conjugates five times into the femoral muscle at two-week intervals. First immunization was performed using an emulsified mix of 100 µg vimentin peptide-KLH conjugates plus an equal volume of Freund’s complete adjuvant (Sigma, USA), while in second to fifth injections, 50 µg KLH-conjugated peptide and incomplete Freund’s complete adjuvant were put to use. Three days before cell fusion, an additional injection of 20 µg of KLH-peptide conjugate without adjuvant was performed intravenously.
To monitor the mice immunization, each mouse was bled with a vertical tail vein incision before each immunization and also before the cell fusion. The obtained sera were subjected to use in an ELISA assay to determine the titer of the antibody; as described below: first, a 96-well ELISA plate was coated with 50 ul of 10 µg/ml concentration of peptide and incubated at 4°C overnight. The next day, the wells were washed with 1×PBS, then were blocked with 200 ul of 3% BSA for 3 hr at Room Temperature (RT). Subsequently, the mice sera -in a serial dilution starting at 1/500 were added to the wells, followed by incubation of the plate for 1.5 hr at RT, then washing and re-incubation by rabbit anti-mouse HRP-conjugated antibody (1:2000) (Padzaco, Tehran, Iran) for additional 1 hr, where washing by 1×PBS was then repeated according to above manner. At the final step, 100 µl of Tetramethylbenzidine (TMB) chromogen substrate (Sigma, USA) was added, and the plate was moved to a dark place for 15 min. Finally, the reaction was stopped with 50 µl of 0.16 M H2SO4; afterward, the optical density was measured using a microplate reader instrument (BioTek, USA).
According to our previous reports, the mouse with a higher serum antibody titer was subjected to hybridoma production by fusing its splenocytes to the myeloma SP2/0 cell line 22. Briefly, the spleen cells of the hyperimmunized mouse were resected and its cells were washed out using a small syringe and 10 ml 1×PBS in a 10 cm Petri dish and collected in a 15 ml falcon tube. After two times washing with 1×PBS, cells were counted and 2×107 B cells plus 5×107 SP2/0 mouse myeloma cells were mixed and fused using 1 ml of PEG solution (P7181, Sigma) adding dropwise to the mixed pellet with a very gentle vibration. HAT medium was used for selection of fused (hybridoma) cells. A hybridoma clone with a high affinity to vimentin immunogen peptide was determined by performing ELISA and subjected to clone expansion as well as antibody production and purification.
The study was approved by Avicenna Research Institute Ethical Committee (IR.ACECR.AVICENNA. REC.1400.004) and Kurdistan University of Medical Sciences Ethical Committee (IR.MUK.REC.1398.002). All animal experiments were performed according to animal ethics guidelines.
Monoclonal antibody purification by affinity chromatography: For monoclonal antibody purification, the supernatants of the obtained hybridoma cell culture were filtered using 0.45 µm filters (Nalgene, USA). They were then passed through an affinity column prepared by coupling CNBr-activated Sepharose 4B (Sigma, USA) to the vimentin peptide immunogen described previously 23. The purity of the antibody and its reaction with the vimentin peptide were assessed by SDS-PAGE and ELISA, respectively, as described above.
Cell lines: All cell lines including NCCIT (RRID: CVCL_ 1451), HeLa (RRID: CVCL_0030), MDA-MB-231 (RRID: CVCL_0062), HFFF-PI 6 (RRID: CVCL_ 9V94), PaCa2, CHO, U373, ESTDAB-75, 4T1, CT26, GL261, COS7, and A172, MDA-MB231, BT-474, MCF-7, Hep-G2, LCL, Jurkat, HT-29, HT-1080 were obtained from National Cell Bank of Iran (NCBI, Tehran, Iran) and bovine Sertoli cells (Avicenna research Institute, Tehran, Iran).
Immunoprecipitation-Western blotting (IP-WB): The purified anti-vimentin antibody was employed for immunoprecipitation of vimentin from U373 cell lysates using protein A-Sepharose bead according to the protocol described elsewhere 24. Briefly, 20 ul of protein A-Sepharose bead added to a 500 ul of cell lysate (pH=7.4) and incubated for 1 hr at 4°C. After brief spinning the precipitated phase was washed twice with 1×PBS. The immunoprecipitated cell lysate was subjected to Western blotting by loading on a 6% SDS-PAGE gel (100 V, 2 hr), then by protein transfer onto a PVDF membrane (Millipore, USA) and blocking with 5% non-fat milk. The filter was then incubated with a commercial anti-vimentin antibody (Abcam, USA) for 1.5 hr. After washing by PBS-Tween 0.05% (PBS-T), the filter was incubated this time with HRP-conjugated sheep anti-mouse antibody (1:2500 dilution) (Padzaco, Tehran, Iran) for 60 min, followed by washing and developing with an ECL system (GE Healthcare, Sweden).
Immunocytochemistry (ICC): The produced antibody was evaluated to recognize vimentin protein in Immunocytochemistry (ICC) assay. For this purpose, the cells were cultured on 8-well laminated glass slides (Marienfeld, Germany) in RPMI (Gibco, USA) medium and were incubated at 37ºC with 5% CO2 atmosphere and 98% humidity conditions overnight. The next day, the slides were washed with Tris-Buffered Saline (TBS) and were allowed to dry at RT for 15 min. The cells were then fixed and permeabilized by 2 min of incubation in cold acetone (-20ºC).
In the next step, slides were blocked (5% sheep serum for 10 min at RT), then were incubated with the produced monoclonal ant-vimentin antibody at a concentration of 5 µg/ml in TBS with 1% BSA (TBS-BSA) for 1 hr at RT. Subsequently, the slides were washed, and sheep anti-mouse fluorescein isothiocyanate-conjugated (FITC, Padzaco., Tehran, Iran) was added (at a 1:50 dilution). Incubation was continued for 45 min. Consequently, the slides were washed and subjected to 5 min of nucleo-staining by DAPI (1 µg/ml) (Calbiochem, USA), mounted with 80% TBS-glycerol and examination under a fluorescent microscope (Olympus, Japan).
Flow cytometry: Flow cytometry analysis was performed with the intention of CSV detection ability by the produced antibody. To this end, 1×106 cells from MDA-MB231, BT-474, MCF-7, HepG2, LCL, Jurkat, HT-29, NCCIT, and HT-1080 cell lines were obtained by trypsinization and harvesting. In the next step, the cells were used for immunostaining using 10 µg/ml of the produced anti-vimentin antibody and 1/1000 dilution sheep anti-mouse FITC-conjugated antibody as primary and secondary antibodies, respectively, according to the protocols mentioned elsewhere 25. An anti-HIV protein envelope monoclonal antibody (Padzaco, Tehran, Iran) was used as isotype control and a FACSCalibur flow cytometry (Becton Dickinson, USA) and Flomax flow cytometry analysis software (Partec, Germany) was used for sample analysis and data acquisition, respectively.
Protein sequence analysis: The amino acid sequence of vimentin protein was obtained from the UniProt database 26. Multiple sequence alignment for the vimentin sequences in different species was performed using seeded guide trees and Hidden Markov Model protein-profile techniques implemented in the Clustal Omega web server 27.
Results :
Assessment of peptide-carrier portions conjugation: Direct evaluation of peptide-KLH conjugation efficiency by running to the SDS-PAGE gel is not achievable because of high molecular weight of KLH. As an alternative, the peptide-BSA conjugate prepared by the same buffering systems and laboratory circumstances with peptide-KLH conjugate was subjected to 10% SDS-PAGE gel. The change in mobility pattern for peptide-BSA in comparison to BSA, and BSA-linker conjugate (i.e., BSA-MBS) as controls, was demonstrative of the conjugation of immunogen peptide to BSA protein as well as KLH protein (data not shown).
Hybridoma cell generation, clone selection, and antibody purification: The ELISA assay showed the presence of anti-vimentin peptide antibody in the immunized mice sera. The obtained results confirmed the immunization of all mice against vimentin peptide. Hybridoma cells were generated by fusion the spleen of the mouse with the highest anti-vimentin serum antibody level to the SP2/0 cell line. Among the several obtained hybridoma clones, the clone, namely 7C11-D9, that showed the highest reactivity with immunogen peptide in ELISA, was selected and employed for clone expansion and antibody purification (data not shown).
Characterization of the purified monoclonal antibody: The isotype of the selected hybridoma clone (i.e., 7C11-D9) was determined as IgG1 with a light kappa chain using a mouse monoclonal antibody isotyping kit (IsoStrip, Roche, Ind, USA). The affinity-purified monoclonal antibody was evaluated for purification quality by running on SDS-PAGE under non-reducing conditions. The results revealed the presence of a ~150 kDa band representing IgG and demonstrated the high quality of purification understood from the absence of any non-specific or additional band. The purified antibody was also re-reacted with the coated vimentin peptide using ELISA, showing the maintenance of antigen recognition property by the 7C11-D9 antibody after purification (data not shown).
To verify the reactivity of the purified antibody with the corresponding protein (i.e., Vimentin), U373 cell lysate as vimentin-positive was precipitated by 7C11-D9 purified antibody. The immunoprecipitated cell lysate was subjected to Western blot using an anti-vimentin commercial antibody. In figure 1, a ~53 kDa band illustrates the reaction of the commercial antibody with vimentin in the U373 cell line, confirming the reaction of 7C11-D9 with vimentin protein.
In the ICC assay, the produced antibody also showed reactivity with vimentin protein expressed in a panel of cell lines with different origins, including A172 (human brain glioblastoma), MDA-MB231 (Human breast cancer), PaCa2 (human pancreatic adenocarcinoma), U373 (human glioblastoma astrocytoma), COS7, (African green monkey kidney), ESTDAB-075 (human melanoma), GL-26 (mouse glioma), CT-26 (mouse colon carcinoma), CHO (hamster ovarian cell), and Bovine Sertoli cells. As shown in figure 2, the green color represents the interaction between the 7C11-D9 antibody with vimentin and the blue color shows the nucleus of cells stained by DAPI.
The sequence alignment of antigenic peptide and comparison with ICC data shows that antigenic epitope does not contain Alanine and Valine amino acids (MEENFAVEAANYQD) of the immunoigenic peptide (Table 1). The obtained data from flow cytometry analysis revealed that the 7C11-D9 antibody could detect the cell surface expression of vimentin in a series of cell lines, as illustrated in figure 3.
Discussion :
The high rate of death from cancer globally, with nearly 1×107 deaths annually 28, notifies the necessity of developing more effective therapeutic agents. Along these lines, monoclonal antibodies are valuable tools applicable in diagnosing and treating malignancy disorders 29.
In the current work, we produced a monoclonal antibody against human vimentin nominated as clone 7C11-D9 using conventional hybridoma technology. This study used a peptide-based immunization approach by utilizing a synthetic peptide designed from the human vimentin amino acid sequence. Nowadays, producing antibodies against synthetic peptides capable of recognizing their corresponding native protein is a well-accepted approach 30,31. This strategy has several benefits, such as producing a more specific antibody against a sequence of interest, post-translational modification situations, and protein splice variants, vs. generation of antibody by utilizing whole protein or whole-cell as immunogen 32.
In the current work, several hybridoma clones producing anti-vimentin antibodies were obtained within clone 7C11-D9 (IgG1 heavy chain isotype with kappa light chain) showed more reactivity with the immunizing vimentin peptide that led to its selection and employment for antibody purification and more characterization.
The affinity-purified 7C11-D9 antibody could maintain its ability to recognize the vimentin immunizing peptide after purification, assessed by ELISA examination. That was a great result showing desired stability of this antibody to recognize its corresponding immunogen after purification. However, the IP-Western blot result was the most critical data confirming the ability of the 7C11-D9 antibody to recognize vimentin protein, where the produced antibody could precipitate the vimentin protein in U373 lysate as a vimentin-positive cell. This precipitation ability was confirmed using a commercial antibody in western blot analysis (Figure 1). Furthermore, our unpublished results also showed that the 7C11-D9 antibody could precipitate vimentin protein in other cell lines. It could react with vimentin in Western blot analysis with/without precipitation (data not shown). The data from this part suggest the applicability of the 7C11-D9 antibody in IP and Western blot analysis experiments.
In ICC experiments, the purified 7C11-D9 antibody was able to stain vimentin in human, bovine, mouse, hamster, and monkey originated cells (Figure 2), which was an expected data because of the very high sequence homology present in the designed vimentin peptide antigen (Table 1). Based on the sequence analysis of vimentin in different species, we believe the produced antibody can react with vimentin in other non-examined species such as dogs, horses, sheep, chickens, pigs and rats, that this indebted to our peptide immunogen designing form a conserved region of vimentin. The ICC data indicated the usefulness of the 7C11-D9 purified antibody to detect vimentin protein in different origin samples by ICC (Table 1).
Another interesting acquired outcome from ICC was the observation of the different patterns of cellular localization from the nucleus to the cell membrane of vimentin based on green fluorescence signals representing the vimentin expression (Figure 2). This observation aligned with other reports 9 and encouraged us to evaluate 7C11-D9 for CSV detection.
The obtained results from the flow cytometry experiment indicated the ability of the produced antibody in staining CSV in a panel of cell lines, including standard and hematological originated cells, including LCL (Lymphoblastoid cell lines) and Jurkat (acute T cell leukemia), as well as other cancer cells lines with different organ origins including MDA-MBA-123 (breast adenocarcinoma), BT-474 (ductal carcinoma), MCF-7 (breast adenocarcinoma), HepG2 (hepatocellular carcinoma), HT-29 (colorectal adenocarcinoma), NCCIT (pluripotent embryonal carcinoma), and HT-1080 (fibrosarcoma) with varying amounts.
Although the comparison of cell surface expression level of vimentin was not in our aim lines, some findings were engaging in the obtained data from flow cytometry, such as a relatively high level of CSV detection in breast cancer cell line, i.e., MDA-MBA-123 (52%) and BT474 (71%) cells as Triple-Negative Breast Cancer (TNBC) and triple-positive breast cancer cell lines, respectively, and MCF-7 (47.3%) cells, that ties well with other studies in this context [33]. Regardless of recent reports which demonstrate that vimentin could not be a relevant prognostic factor in TNBC patients 34, in the other studies, it has been shown that vimentin overexpression is related to poor prognosis in breast cancer patients 35-38. Therefore, the relatively high level of cell surface vimentin expression in all breast cancer cell lines (MDA-MBA231, BT-474, and MCF-7) might introduce anti-vimentin monoclonal antibodies as a therapeutic candidate for breast cancer therapy.
According to our findings, the high level of CSV detection was not limited to breast cancer cell line, and the produced antibody could detect CSV in other non-breast cancer cells (i.e., Jurkat (90.6%), LCL (84.8%), HT-1080 (48.6%), HT-29(37.3%), HepG2(49.3%). NCCIT (76.1%), as illustrated in figure 3, might be introductive of CSV as a target in other cancer cell types as reported by others 39,40. The data from this part indicated the capability of the produced antibody to use it as a tool for phenotyping of cancer samples by cell surface immunostaining methods.
Regarding the therapeutic potency of anti-vimentin antibodies, the recent therapeutic benefits of monoclonal antibodies against glioblastoma multiform expressing CSV have been recently demonstrated 41,42. Therefore, the production of the 7C11-D9 antibody has provided the fortune to evaluate its therapeutic effects against different cancer cells or assess its potency to use it as a tool for antibody-mediated drug delivery systems. Many in vitro and in vivo experiments are needed to this end. However, it should be noted that such experiments have been started by our research group (not published data). In addition to CSV therapeutic targeting potential, as mentioned, it has been demonstrated that surface vimentin expression is a marker for the detection of Circulating Tumor Cells (CTC) as well as Cancer Stem Cells (CSC) 4,43-45. This characteristic also indicates that the 7C11-D9 antibody can be valued for the characterization and detection of CTC and CSC.
Conclusion :
Taken together, 7C11-D9 anti-vimentin monoclonal antibody can be used as an immune diagnostic tool. It might be evaluated as an immune therapeutic tool where detection or targeting of vimentin is needed.
Acknowledgement :
This work was supported by grants from Avicenna Research Institute, ACECR, Tehran, Iran (IR.ACECR.AVICENNA.REC.1400.004) and Deputy for Research and Development of Iran Ministry of Health and Medical Education, Tehran, Iran (IR.MUK.REC.1398.002). The authors are thankful to the staff of Avicenna Research Institute for their assistance during this project and also from the Deputy for Research and Development of Kurdistan University of Medical Science.
Conflict of Interest :
There is no conflict of interest in this study.
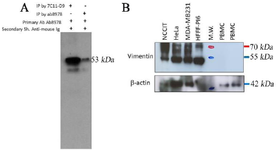
Figure 1. A) The results of immunoprecipitation and Western blot (IP-WB) analysis. The produced monoclonal antibody 7C11-D9 (Lane 1) and commercial anti-vimentin antibody (RV-202, Abcam) (Lane 2) were used for precipitation of vimentin in U373 (Lane 2, 3) as vimentin positive. Commercial anti-vimentin antibody and HRP-conjugated sheep anti-mouse were used as primary and secondary antibodies, respectively.) A band of ~53 kDa is represented the 7C11-D9 mediated immunoprecipitated vimentin protein detection by anti-vimentin antibody in U373 cells. B) Western blot analyses of a HRP-conjugated anti-vimentin antibody (7C11-D9) on cell lysates from different cancer cell lines and PBMC from normal healthy individuals. NCCIT (RRID: CVCL_1451), HeLa (RRID: CVCL_ 0030), MDA-MB-231 (RRID: CVCL_0062), HFFF-PI 6 (RRID: CVCL_9V94), M.W. (Molecular weight marker). Similar filter membrane was probed with mouse monoclonal anti-beta actin antibody as primary antibody as internal loading control. HRP-con-jugated sheep anti-mouse Ig was used as secondary.
|
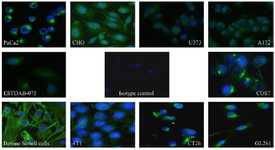
Figure 2. The Immunocytochemistry (ICC) assay results using the produced anti-vimentin (7C11-D9) and different cells with different species and tissue origin. Sheep anti-mouse FITC conjugated was used as a secondary antibody, and DAPI was employed for nucleus staining. The green fluorescence represents the interaction of the 7C11-D9 antibody the blue color represents the nucleus. A fluorescent microscope with a 40× magnification was used for visualization.
|

Figure 3. Flow cytometry results of Cell-Surface Vimentin (CSV) detection in different cells. The produced anti-vimentin 7C11-D9 and sheep anti-mouse FITC conjugated were primary and secondary antibodies, respectively. An anti-HIV antibody was used as isotype control.
|
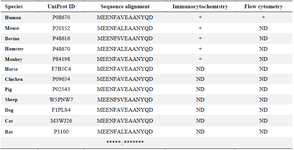
Table 1. Multiple sequence alignment results for the selected vimentin peptide in different species as well as the summary of immunoassay results for the produced anti-vimentin antibody (7C11-D9)
ND: Not done
|
|