Formulation, Characterization, and Evaluation of Wound Healing Potency of a Novel Mattan tailam Nanogel Based on a Famous Traditional Siddha Formula
-
Sakthiganapathi , Meenachisundaram
-
Department of Pharmacognosy, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, A Government of Puducherry Institution, Gorimedu, Puducherry, India
-
School of Pharmacy, Sri Balaji Vidyapeeth (Deemed to be University), Puducherry, India
-
 Prakash Yoganandam , Gnanakumar
College of Pharmacy Mother Theresa P.G & RI of Health Sciences A Government of Puducherry Institution Gorimedu, Puducherry 605 006, India, Tel: +91413 2271200; E-mail: gprakashyoga@gmail.com
Prakash Yoganandam , Gnanakumar
College of Pharmacy Mother Theresa P.G & RI of Health Sciences A Government of Puducherry Institution Gorimedu, Puducherry 605 006, India, Tel: +91413 2271200; E-mail: gprakashyoga@gmail.com
-
Department of Pharmacognosy, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, A Government of Puducherry Institution, Gorimedu, Puducherry 605 006, Puducherry, India
-
Gopal , Venkatachalam
-
Department of Pharmacognosy, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, A Government of Puducherry Institution, Gorimedu, Puducherry 605 006, Puducherry, India
Abstract: Background: The Mattan tailam mixture has been extensively used to heal ulcerous wounds in traditional Siddha practice. The present study aimed to synthesize a Mattan tailam nanogel and evaluate the enhancement of wound healing potential in an experimental wound model.
Methods: Mattan tailam nanogel was synthesized using the high-energy milling approach, and characterization of nanogel and potency of wound healing was investigated. The novelty of this study was the nanogel preparation of Mattan tailam.
Results: As expected, a synthesized novel nanogel of Mattan tailam has a distinct, prominent peak with a spherical form, is negatively charged and has an average particle size of 20–30 nm. Mattan tailam nanogel treated rats showed a remarkable reduction (p<0.001) in the wound area. On the 16th day, 10% Mattan tailam nanogel treatment resulted in a higher percentage of wound contraction. The 10% Mattan tailam nanogel group exhibited a faster epithelialization time (14.33 days) and a greater hydroxyproline concentration than the others. The topical application of 10% Mattan tailam nanogel increased tensile strength, signifying a better therapeutic indication.
Conclusion: The present findings prove that polyherbal Mattan tailam nanogel formulation significantly improves collagen production, wound contraction, and tensile strength.
Introduction :
The wound-healing process is repairing a wound that has been damaged and inflicted on the skin and other soft tissues 1-3. After the injury, there is an inflammatory response, and the cells under the dermis increase collagen production in the connective tissue 1-3. Later, the outer skin of the epithelial tissue is regenerated 1-3. Inflammation, proliferation, and remodeling are the three stages of wound healing 4. The proliferative phase includes angiogenesis, collagen deposition, granulation tissue formation, epithelialization, and wound contraction 5,6. Endothelial cells produce new blood vessels during the process of angiogenesis 5,6. Fibroblasts release collagen and fibronectin to synthesize a temporary extracellular matrix in fibroplasia and granulation tissue 7,8. Epithelial cells then crawl across the wound bed to cover it, and the wound is contracted by myofibroblasts, which grasp the edges of the wound and contract using a mechanism like that in smooth muscle cells 9,10.
Plants or plant-derived chemical entities must be identified and formulated to treat and manage wounds 11,12. Several herbal products are currently being studied in this direction. Various herbal products have been used to treat injuries over the years 11,12. Nanogel formulation is a versatile platform for enhancing herbal properties 13. Herbal nanogels transform natural products into the most suitable drugs for treating various diseases, including cancer, skin diseases, and diabetes 13,14. Compared with oral medications, herbal nanogel has fewer side effects relating to the patient's compliance 13,14. Although many natural medicinal products have been developed, some are highly toxic, interact with conventional drugs, and have adverse side effects 13,14. In recent years, herbal products have been accepted in modern medical systems, and the quality of herbal products needs to be evaluated. Herbal nanogel formulations are expected to provide synergistic effects at low drug concentrations with no adverse effects in the pharmaceutical industry 13,14. Herbal nanogel products can be a practical new drug carrier system.
Nanogels can meet all the basic requirements of adaptive nanocarrier delivery media. Nanogels are mainly used in nanomedicine applications as a new drug carrier for response-based treatments 15. Nanogels are ionic or non-ionic nanoparticles composed of physically or chemically cross-linked spolymers, which can be hydrophilic, hydrophobic, or amphiphilic 16,17. The new drug carrier should have two main features: delivering the drug at the required rate and effectively delivering the drug to the site of action. Therefore, nanogels have many advanced functions, which can be equivalent to the demand for modern medicines 18,19. Nanogels are used for local and systemic drugs due to their inherent swelling properties, which are attributed to their chemical modification to help release the drug in the desired dosage form 20. Nanogels have important functions, such as enhancing drug absorption across physiological barriers and sustained drug release. Nanogels can easily formulate skin patches, biosensors, and ionic drug delivery 21.
Mattan tailam, a herbal-mineral classic Siddha formulation, is used to heal suppurating wounds and is very useful in healing diabetic ulcers 22. Medicated oil is made by boiling base oil with the juice of prescribed herbal and mineral medicines for a long time until it is dehydrated or almost dehydrated. This process results in the transfer of some therapeutically active ingredients into the base oil 22,23. The traditional method of tailam preparation ensures that the oil is enriched with active ingredients 22,23. Mattan tailam is a herbal-mineral Siddha formulation traditionally used for various conditions such as eczema, oozing eczema, itching, sores, chronic ulcers, bedsores, anal fistulas, ear infections, carbuncle ulcers in diabetes, anal abscesses, non-healing external ulcers, folliculitis, alopecia, and burns 22. Therefore, the present study was modernized to prepare the herbal nanogel with a famous traditional Siddha formulation, Mattan tailam, and evaluate the effectiveness of the herbal nanogel against the wound healing process and compare it with conventional and allopathic formulations.
Materials and Methods :
Raw materials: The raw materials, individual ingredients, and traditional formulation of Mattan tailam were purchased from the local drug market and identified by Siddha's physician in August 2019. Aloe vera gel was freshly prepared using the conventional method. Specimen copies (#MTPGRIHS/PCOG/2019/110) have been de-posited with the Department of Pharmacognosy, College of Pharmacy, Mother Theresa Post Graduate and Research Institute of Health Sciences, A Government of Puducherry, Puducherry, India for future reference. Ingredients of a traditional Siddha formulation, Mattan tailam (Paccai enney), for wound healing are shown in table 1.
Synthesis of Mattan tailam nanoparticles: Nanoparticles were synthesized using the high-energy milling method with slight modification 24. First, the ingredients of Mattan tailam were well ground with a blender grinder to make fine powders. The fine powders obtained were ground for 1 hr using a 20 mm ball (Zirconia) using a ball mill (PM100; Retsch, Germany) to obtain nanoparticles. Then, the obtained nanoparticles (almost 5 mg) were ground in a ball mill (10 mm balls: 300 rpm) for 15 hr. All nanoparticle samples obtained were used for nanogel preparation.
Preparation of Aloe vera gel: The previously described procedure was used to extract Aloe vera gel 25. Aloe vera gel was prepared from Aloe vera plant leaves, which were collected and processed at the Department of Pharmacognosy, Mother Theresa Post Graduate and Research Institute of Health Sciences, Puducherry. The leaves were thoroughly washed, and the Aloe vera gel was extracted by cutting through the parenchymal membrane of the leaves. Aloe vera gel was filtered and refined with glycerine and triethanolamine, yielding a 95% pure Aloe vera gel. The resultant gel was then heated for 3 min at 50°C, cooled, and kept at 5°C for later use.
Formulation of Mattan tailam nanogel: Herbal nanogel was prepared using Mattan tailam and Aloe vera gel with the modified method 25. In preparing topical nanosuspension-based gel formulation, 5%, and 10% Mattan tailam nanoparticles and 1% Aloe vera gel were incorporated into 0.5% carbomer 940 gel base 0.1% sodium salt of methylparaben (as a preservative). The ingredients were mixed under continuous stirring using a high-speed mechanical stirrer (Remi motor) to achieve the required viscosity. An aqueous dispersion of 5% and 10% Mattan tailam nanogel was added gradually to the resulting mixture with continuous stirring; then the pH of the developed nanogel was neutralized using a drop of 0.3% ammonia solution. Based on the dose range-finding pilot study (not published), 5% and 10% Mattan tailam nanogels were chosen for the formulation and experimental
analysis. These doses were compared to the wound healing potential of Mattan tailam.
Characterization of Mattan tailam nanogel: The structural and morphological properties of the nanogel were analyzed using the following analytical methods. Ultraviolet-visible spectrometer (BioSpec Nano, Shimadzu) and Fourier Transform Infrared (FT-IR) spectroscopy (IR Affinity S1, Shimadzu) was used to analyze the surface plasmon resonance of the Mattan tailam nanogel. The Zeta potentials of Mattan tailam nanogel were measured with a Zetasizer 3000 instrument (Malvern ZEN-1690). X-ray Diffraction (XRD) patterns of the Mattan tailam nanogel were analyzed on the X-ray diffractometer (Xpert-Pro). The morphological properties of the elemental compositions of the Mattan tailam nanogel, including the size and shape, the uniformity of the nanogels, and the dispersibility, were identified by Field Emission Scanning Electron Microscopy (FE-SEM) (TESCAN MIRA3), Transmission Electron Microscope (TEM) and Energy Dispersive X-ray Analysis (EDAX). All measured variables were carried out in triplicate at room temperature for error analysis.
Experimental rats: The study design and experimental protocol conform to the Institutional Animal Ethics Committee and have been approved with an Ethical Clearance Number: CBLRC/IAEC/04/01-2021. Female albino Wistar rats weighing 200-220 g were used for this study. All rats were housed in polypropylene cages, six rats per cage, and fed a standard pellet diet and water ad libitum. All rats were maintained at a temperature of 23°C, 55-65% humidity, and 12 hr light/12 hr dark with automated light cycles. All rats were weighed weekly throughout the experiment. The rats were anesthetized prior to the infliction of the wounds, and the surgical procedures were performed in sterile environments. All rats were closely monitored for infection, and rats showing signs of infection were excluded from the study and replaced.
Acute toxicity study: For acute oral toxicity testing, OECD guideline 423 was followed. The rats were fasted overnight and given unlimited water. Three rats in each group received an initial dose of 5 mg/kg extract orally. If two or three rats died, the dose administered was referred to as toxic. The same amount was given to three other animals, when one rat died to confirm the toxic dose. If no mortality occurred, the treatment was repeated with larger doses of 50, 300, and 2000 mg/kg body weight. The rats were observed after administration at least once during the first 30 min, occasionally during the first 24 hr, with particular attention during the first 4 hr and daily for the next 14 days. Moreover, cytotoxicity of the Mattan tailam was performed in our laboratory in the earlier study 26.
Wound-healing activity: Excision and incision wound models were used to compare the wound-healing abilities of Mattan tailam traditional and updated herbal nanoformulations.
Excision wound model: The rats were anesthetized before and during the creation of the wounds. As described by Morton and Malone 27, excision wounds were inflicted on the rats. The rats' back fur was shaved with an electric clipper, and the expected area of the wound was created as outlined on the animals' backs with methylene blue using a circular stainless steel template. A full-thickness excision wound with a circular area of 500 mm2 and a depth of 0.2 cm was created along the markers using serrated forceps, a surgical blade, and sharp-pointed scissors, and the entire wound was left open.
The rats were divided into five groups of six each. Group 1 rats received topical application of Aloe vera gel as a placebo control. Group 2 rats received topical application of 5% Mattan tailam's nanogel until complete epithelization. Group 3 was treated topically with 10% Mattan tailam's nanogel until complete epithelialization. Group 4 was treated topically with traditional Mattan tailam until complete epithelization. Group 5 was treated topically with Nitrofurazone ointment (0.2% w/w). The wound closure rate was determined by tracing the wound with transparencies and a permanent marker on days 0, 2, 4, 8, 12, 16, 18, and 20 after the wound was created. Graph paper was used to mea-sure the wound areas that were recorded. The epithelization duration was determined by the number of days it took for the scab to peel off without leaving a raw wound. Based on the dose range-finding pilot study (not published), 5% and 10% Mattan tailam nanogels were chosen for the formulation and experimental ana-lysis. 10% Mattan tailam topical applications showed wound healing potential in excision wound model as compared to 5% Mattan tailam.
Incision wound model: As in the model above, the rats were anesthetized before and during the creation of the wound. The back fur of the rats was shaved with an electric hair clipper. A 6 cm long paravertebral longitudinal incision was created through the skin and dermal muscle on the back, as described by Ehrlich and Hunt 28. After the incision, sutures were placed on the cut skin at 1 cm intervals. The wounds were left undisguised. The rats were divided into five groups of six each. Group 1 rats were treated topically with the Aloe vera gel base as a placebo control. Group 2 rats were treated topically with 5% Mattan tailam's nanogels until complete epithelization. Group 3 was treated topically with 10% Mattan tailam's nanogel until complete epithelialization. Group 4 was treated topically with traditional Mattan tailam until complete epithelization. Group 5 was treated topically with Nitrofurazone ointment (0.2% w/w).
The sutures were removed eight days after the wound, and treatment continued. Skin tear strength was measured on day 10 using the method described by Lee 29. The anesthetized rats were placed on the table, and on each side of the wound, a line was drawn 3 mm from the line. This line was grasped at each opposite end using forceps. One of the tongs was fixed, the other articulated on a freely suspended light metal plate. The weight was gradually increased, and the wound's edges were gradually pulled apart. Weight addition was stopped when the wound began to open and the weights added were recorded as a measure of fracture toughness. Three readings were taken for each incision. The mean value of the group was taken as the individual breaking force value. The mean indicates the breaking strength for a specific group. Based on the dose range-finding pilot study (not published), 5% and 10% Mattan tailam nanogels were chosen for the formulation and experimental analysis. 10% Mattan tailam topical applications showed wound healing potential in incision wound model as compared to 5% Mattan tailam.
Statistical analysis: All data were presented as mean±SEM (n=6). Comparisons of determinant variables between all pooled data for all parameters were analyzed statistically by one-way ANOVA followed by Tukey-Kramer multiple comparisons using the SPSS software package, version 15.01 for windows, and statistical significance was defined as p<0.05, p<0.01, and p<0.001.
Results :
Characterization: UV spectroscopic analysis was examined for the Mattan tailam nanogels in a range of 200-800 nm. FT-IR was used to identify the structural features and selective phytochemical components. The results have an estimated range of 4000-400 cm−1. Mattan tailam nanogels were exposed to CuK1 X-ray diffractometer radiation (λ=1.5406 A°) at 40 kV and 30 mA with a 2θ range. Mattan tailam nanogels were placed on the sample holder and sputter-coated with gold. Then, the average particle size and shape of the Mattan tailam nanogels were studied by FE-SEM (Japan). The size and shape of these Mattan tailam nanogels are studied using a TEM at 100 kV.
UV - Vis spectroscopic analysis: The development of Mattan tailam nanogels was first analyzed using UV spectroscopy in the 200-800 nm range. The absorption spectra of green-produced Mattan tailam nanogels displayed a characteristic peak of 250, 300, and 300 nm, F-1, F-2, and F-3, respectively.
FT-IR spectroscopic analysis: Mattan tailam nanogels were analyzed using an FT-IR as an affirmative method, which provides an imprint of present molecules' vibrational and rotational modes, assisting in classifying functional and prospective plant chemicals involved in reducing Mattan tailam nanogels. Figure 1 shows the FT-IR graph representing the functional groups present on the Mattan tailam nanogels. The peaks at wavenumber at 3277.06 cm-1, 1633.71 cm-1, 1105.21 cm-1, and 678.94 cm-1 were observed. The band around 3277.06 cm-1 was associated with the characteristic absorption peak of –CH stretching. The band around 1633.71 cm-1 was associated with the characteristic absorption peak of –primary or secondary amide. The band around 1105.21 cm-1 was associated with the characteristic absorption peak of –C–O stretching. The band around 678.94 cm-1 was associated with the characteristic absorption peak of saccharide structure.
XRD analysis: The structure of the Mattan tailam nanogels was indicated by XRD analysis of the synthesized CuNPs. At 2θ values of F-1 (25.5, 28.02, 30.00), F-2 (24.6622, 25.8326, 28.0072, 31.8254) and F-3 (25.00, 67.00, 27.00) degrees, diffraction peaks were observed. Figure 2 shows the XRD analysis of synthesized nanoparticles.
EDAX analysis: Figure 3 shows the elemental analysis of the Mattan tailam nanogels according to the EDAX spectrum of the FE-SEM image. The molecular mass percentage and atomic value of elements in the nanogel were consistent. The data generated by EDAX analysis showed three spectra peaks corresponding to the elements making up the true composition of the Mattan tailam nanogels. Elemental mapping of a Mattan tailam nanogels and image analysis confirmed the presence of copper.
ZETA sizer: Synthesized Mattan tailam nanogels performed zeta size analysis to measure the size of the particles using the ZETA sizer. The zeta potential mainly depends on the surface charge, and is critical for the stability of Mattan tailam nanogels in suspension, and is also a key determinant in their first adsorption on the cell membrane. After adsorption, the endocytic uptake rate depends on the particle size. Figure 4 shows the corresponding particle size of nanoparticles as visualized by the ZETA particle size analyzer. The average diameters of Mattan tailam nanogels compositions were 122±8 and 736±2 nm, respectively. However, Mattan tailam size significantly increased (p<0.001) when Aloe vera gel was used at the same weight ratio compared to Mattan tailam.
FE-SEM analysis: The SEM examination was performed using Schottky FE-SEM (Japan) model TESCAN MIRA3 LMH. The structure and scale of Mattan tailam nanogels were determined using FE-SEM analysis. Under the FE-SEM microscope, Mattan tailam nanogels were found to be nanoscale (500 nm) in particle size, spherical, and uniformly dispersed. Figure 5 shows an SEM image indicating the formation of variable sizes of nanogels.
TEM analysis: Mattan tailam nanogels were examined through TEM (JEOL 2010, Tokyo, Japan) following the recommended procedures 30. A drop of Mattan tailam nanogels was mounted over the carbon-coated copper grid of TEM. The solvent was air-dried at room temperature, and then the sample was analyzed for its size and shape at an accelerating voltage of 200 kV (Figure 6).
Excision wound model: A statistically significant increase in wound-healing activity was observed in the rats treated with the herbal nanogel compared to the traditional formulation and nitrofurazone treatment. In the excision wound model, herbal nanogel-treated rats revealed a significant reduction in the wound area (p<0.001) (Table 2). The 10% herbal nanogel exhibited that the percentage of wound contraction on the 16th day was higher than the traditional formulation and nitrofurazone treatment groups. The herbal nanogel showed a significantly less epithelialization period (14.33 days) in an excision wound model than the traditional formulation and nitrofura-zone (Table 2). The epithelialization period following 10% Mattan tailam nanogels treatment was significantly less (p<0.01) as compared to the respective control group. The epithelization period was also higher in traditional formulation-treated rats, followed by nitrofurazone-treated rats and 5% nanogel-treated rats.
No statistically significant differences between the dry weight of tissues among herbal nanogel, traditional formulation, and nitrofurazone treatment groups were observed (Table 3). However, the hydroxyproline content was higher in the 10% herbal nanogel treated group than in the traditional formulation and nitrofurazone treatment groups (Table 3). Figure 7 shows the photographs of contraction rate and percent wound contraction area on different post-excision days at different time intervals in rats' excision wound model.
Incision wound model: Table 4 shows the effects of the herbal nanogel and traditional Mattan tailam on wound tensile strength in rats inflicted with incision wounds. A significant increase in the wound-breaking strength was observed in the incision wound model with 10% of the herbal nanogel as compared to the herbal nanogel.
Histopathological analysis: Histological investigations of excision wound tissue were done on the 16th day, with histopathological features of tissue from all groups of animals shown in Figure 8 (A-E). Inflammatory cells, collagen fibers with scar tissue, fibroblast cells, and blood vessels were seen in Group I rats (Figure 8A). There was less cellular necrosis in Group IV rats and more collagen fibers and blood vessels (Figure 8B). Group V rats showed prominently increased fibroblast cells, blood vessels, and well-organized collagen fibers as compared to the control (Figure 8C). Necrotic cells with fewer collagen fibers and blood arteries were seen in Group II rats (Figure 8D). More fibroblast cells with collagen fibers, blood vessels, and fewer inflammatory cells were seen in Group III rats, indicating complete tissue regeneration (Figure 8E).
Discussion :
The present study demonstrated that the herbal nanogel formulation of Mattan tailam was successful using a high-energy milling method and natural gel. All characteristics, including structure, functional groups, size, shape, charge, and morphology of the Mattan tailam nanogel, were studied. Mattan tailam nanogel was shown to have three distinct noticeable peaks with negatively charged particles, with an average particle size of 20-30 nm and a spherical shape. Excision and incision wounds were used to assess the wound healing potential of the Mattan tailam nanogel. The efficacy of topical therapeutic application of Mattan tailam nanogel in an excision and incision rat model was most effective.
Siddha is a herbal-based therapeutic technique that dates back thousands of years. With recent developments in biotechnology, traditional Siddha medical systems have enjoyed a considerable rebirth worldwide. Hence, we chose a well-established rodent model to investigate the wound healing ability of the Mattan tailam nanogel. The novelty of the study in comparison to the previous research by Arunadevi 22 was mainly nanogel preparation of Mattan tailam. Moreover, the present study was performed with both incision and excision wound healing experimental and wound dressing designs. Moreover, cytotoxicity of the Mattan tailam was performed in our laboratory in the earlier study, which indicates that Mathan tailam is non-mutagenic and non-cytotoxic, and it is potentially safe to use 26.
Nanomedicine is a broad field of drug delivery that includes a wide range of effective formulations for treating various diseases 31,32. Many illnesses necessitate a certain timing for medicine delivery to achieve maximal effectiveness 31,32. Due to their unique qualities like selectivity, non-specific target, and minimized side effects, nanoparticles and nanogels can be critical tools for overcoming these obstacles and constraints 33,34. In the last two decades, the formulation of nanogels has been the most promising technology in medical research 33,34. According to a recent study, ZnO nanogels offer many potentials for treating superficial microbial skin diseases. ZnO nanogels offer unique qualities such as non-toxicity and low cost, making them ideal for usage as a gas sensor and energy harvesting devices such as solar cells, veristor, pigments, and cosmetics 35,36. Understanding potential toxicological effects after exposure require the characterization of nanoparticles and nanogels. Nanoparticles and nanogels must be characterized using proper analytical methods to determine their size, shape, surface area, morphology, and charge 16,18,20. We used FTIR, XRD, ZETA-Sizer, EDAX analysis, FE-SEM, and TEM to characterize the herbal-based nanogel of Mattan tailam. The size of the nanogels affects their toxicity, and the more extensive specific surface area allows them to enter cells and circulate more quickly. Mattan tailam nanogel was shown to have three distinct noticeable peaks with negatively charged particles, with an average particle size of 20-30 nm and a spherical shape. According to our research, Mattan tailam nanogel has suitable drug delivery methods to provide a platform for successful administration.
Wound healing follows a four-phased process (hemostasis/coagulation; inflammation, migration, proliferation; epithelization; and remodeling) 37,38. The multifactorial coordination of overlapping cellular processes is required for wound healing 37,38. These multifactorial activities affect clotting, phagocytosis, chemotaxis, mitogenesis, angiogenesis, collagen production, extracellular matrix development, and soluble mediators 37,38. Contraction, closure, and re-epithelialization are all essential steps in wound healing 39-41. The type of injury significantly impacts how quickly a wound heals 39-41. Excision, incision, burn, and dead space wound models are all affected differently during the healing process 42,43. Wound induction is minimal in the excision wound model, and the percentage of wound contraction and duration of epithelialization were measured 42,43. In the herbal nanogel-treated rats, we noticed a substantial reduction in wound area (p<0.001). On day 16, the percentage of wound contraction was higher in the Nitrofurazone treatment than in the traditional formulation. Numerous studies have found that the pace of contraction and the duration of epithelization are essential factors in wound healing 42,43. These two factors aid wound closure by lowering the extracellular matrix and limiting keratinocyte migration distance. The 10% Mattan tailam nanogel groups, in particular, had a shorter epithelialization duration (14.33 days). According to the literature, the epithelization stage entails migrating new epithelial cells into infected wound beds 42,43. The epithelization period in the 10% Mattan tailam nanogel groups was brief, possibly due to the vitality of the epithelial cells. Furthermore, the wound contraction and epithelialization effects of the 10% Mattan tailam nanogel were accountable for significant and influential healing. Based on the dose range-finding pilot study (not published), 5% and 10% Mattan tailam nanogels were chosen for the formulation and experimental analysis. 10% Mattan tailam topical applications showed wound healing potential in both excision and incision wound models as compared to 5% Mattan tailam.
Hydroxyproline content measures collagen turnover throughout the wound-healing process and assesses wound-healing therapy quality 42,44,45. The hydroxyproline concentration in the 10% Mattan tailam nanogel treated groups was significantly greater than in the standard formulation and Nitrofurazone groups. In the proliferation phase of the wound healing process, an increase in hydroxyproline content is directly proportional to collagen turnover 42,44,45. The presence of a significant rise in hydroxyproline content in the group treated with 10% Mattan tailam nanogel could imply fibroblast proliferation and migration, resulting in wound constriction and closure.
Despite the lack of advanced treatment and awareness, a 1984 clinical study 46 revealed a lot about the wound healing property of Mattan tailam in diabetic cases, where the chronic ulcerative wound showed its quick healing potential ranging from one to three months depending on the depth and affected area of the ulcer 22. Another evidence of Mattan tailam's preventative activity against chronic wounds persists and progress toward cancer. In addition, the leaf extract efficiently suppresses the mycelia growth of the ringworm fungi Epidermophyton floccosum, Trichophyton mentagrophytes, and Microsporum gypseum. Literature re-search and traditional claims state that Mattan tailam possesses antispasmodic, analgesic, and antiseptic pro-perties 47,48.
The wound healing therapeutic efficacy of 10% Mattan tailam nanogel was further assessed in an incision model by measuring wound tensile strength. We found that treatment with 10% Mattan tailam nanogel increased tensile strength, suggesting a better therapeutic indication than the traditional formulation and Nitrofurazone. Previous studies indicate that wound healing stability or tensile strength mainly depends on collagen turnover and stabilized fibers influenced by hydroxyproline content 49,50. Consistent with these results, the increased wound tensile strength of 10% Mattan tailam nanogel directly involved the higher hydroxyproline levels in the 10% Mattan tailam nanogel treated groups.
Conclusion :
In conclusion, the current findings show that Mattan tailam nanogel formulation developed was successful, and its properties were investigated (structure, functional groups, size, shape, charge, morphology). The topical therapeutic applications of Mattan tailam nanogel in the excision and incision rat models were most effective. The extensive pharmacological and biological actions of the Mattan tailam nanogels comprised of polyherbal composition could significantly affect collagen synthesis, wound contraction, and wound tensile strength. Further research is warranted to enhance therapeutic approaches related to clinical trials.
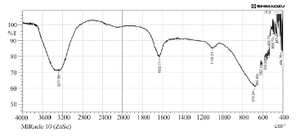
Figure 1. FT-IR graph represents the functional groups present on the Mattan tailam nanogels. The peaks at wavenumber at 3277.06 cm-1, 1633.71 cm-1, 1105.21 cm-1, and 678.94 cm-1 were observed.
|
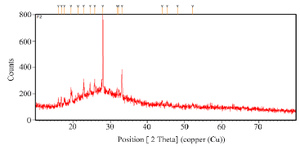
Figure 2. XRD analysis of synthesized Mattan tailam nanogels.
|
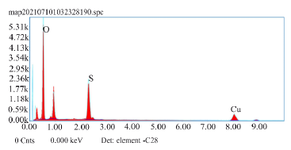
Figure 3. The data generated by EDAX analysis showed three spectra peaks corresponding to the elements making up the true composition of the Mattan tailam nanogels. Elemental mapping of a Mattan tailam nanogels and image analysis confirmed the presence of copper.
|
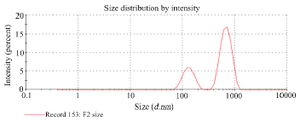
Figure 4. The corresponding particle size of Mattan tailam nanogels as visualized by ZETA particle size analyzer. The average diameters of Mattan tailam nanogels compositions were 122±8 and 736±2 nm, respectively. However, Mattan tailam size significantly increased (p<0.001) when Aloe vera gel was used at the same weight ratio compared to Mattan tailam.
|

Figure 5. SEM image indicates the formation of variable sizes of nanoparticles.
|

Figure 6. The synthesized nanogels were spherical in shape with an average size of around 19.73 nm, revealed by TEM.
|
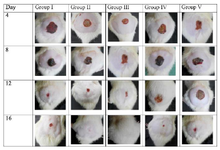
Figure 7. Photographic representation of contraction rate showing percent wound contraction area on different post-excision days at different time intervals in excision wound model in experimental groups. Group I placebo control rats. Group II rats were treated with 5% nanogel, and Group III rats were treated with 10% nanogel. Group IV rats were treated with a traditional formulation, and Group V rats were treated with 0.2 % w/w, nitrofurazone.
|
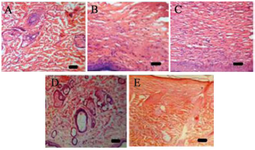
Figure 8. Photomicrograph of histopathological section of wound tissue of rats (stained with Haematoxylin and Eosin, 40x magnification). Inflammatory cells, collagen fibers with scar tissue, fibroblast cells, and blood vessels were seen in Group I (Figure 8A). There was less cellular necrosis and more collagen fibers and blood vessels in Group IV (Figure 8B). Group V showed prominently increased fibroblast cells, blood vessels, and well-organized collagen fibers as compared to the control (Figure 8C). Necrotic cells with fewer collagen fibers and blood arteries were seen in Group II (Figure 8D). More fibroblast cells with collagen fibers, blood vessels, and fewer inflammatory cells were seen in Group III, indicating complete tissue regeneration (Figure 8E). Scale bar: 20 μm in all the figures.
|
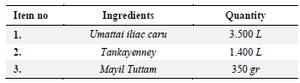
Table 1. Mattan tailam (Paccai enney)-a traditional Siddha formulation for wound healing
Method of Preparation: Dissolve item 3 in item 1, mix with item 2 and boil to prepare tailam.
Important Therapeutic usage: For external application only in Pun, Pilavai and Katil pun Cil vatital. While dressing Punkal (ulcer), use a gauze soaked in this preparation.
|
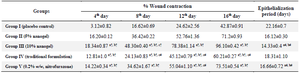
Table 2. Effect of Mattan tailam nanogel and traditional Mattan tailam on % wound contraction and epithelialization period of the wound in an excision wound model
All values are represented as mean±SEM, 𝑛=6 animals in each group. Data were analyzed by one-way ANOVA, followed by Tukey-Kramer Multiple Comparisons Test.
a: significant difference as compared to the vehicle group (group I); b: significant difference as compared to traditional formulation treated group (group II); c: significant difference as compared to standard group (group III), and #p<0.01, †p<0.001.
|
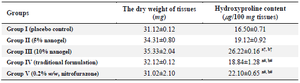
Table 3. Effect of Mattan tailam nanogel and traditional Mattan tailam treatment on tissue hydroxyproline content in an excision wound model
All values are represented as mean±SEM, 𝑛=6 animals in each group. Data were analyzed by one-way ANOVA, followed by Tukey-Kramer Multiple Comparisons Test.
a: significant difference as compared to vehicle-treated (group I); b: significant difference as compared to standard treated group (group II), and #p<0.01, †p<0.001.
|
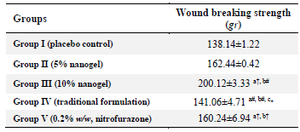
Table 4. Effect of herbal nanogel and traditional Mattan tailam on tensile strength of wound in incision wound model
All values are represented as mean±SEM, 𝑛=6 animals in each group. Data were analysed by one-way ANOVA, followed by Tukey-Kramer Multiple Comparisons Test.
a: significant difference as compared to vehicle treated group (group I);
b: significant difference as compared to traditional formulation treated group (group II); c: significant difference as compared to standard group (group III), and #p<0.01, †p<0.001.
|
|