Comparative Antioxidant and Anti-gout Activities of Citrullus colocynthis loaded Fruit Silver nanoparticles with its Ethanolic extract
-
Karunakaran, Suganya
-
Department of Biotechnology, Dr. MGR Educational & Research Institute, deemed to be University, Maduravoyal, Chennai, India
-
 Hari, Rajeswary
Department of Biotechnology, Dr. MGR Educational & Research Institute, deemed to be University, Maduravoyal, Chennai, India, Tel: +91 9840774705; E-mail: rajihar@gmail.com
Hari, Rajeswary
Department of Biotechnology, Dr. MGR Educational & Research Institute, deemed to be University, Maduravoyal, Chennai, India, Tel: +91 9840774705; E-mail: rajihar@gmail.com
Abstract: Background: The biological synthesis of silver nanoparticles (AgNPs) using plant materials is a rapidly developing method with several alternative medical applications. This comparative study of ethanolic fruit extract of Citrullus colocynthis (C. colocynthis) (EFECC) and synthesized silver nanoparticles (CC-AgNPs) were carried out for antioxidants and anti-gout arthritic activities.
Methods: The AgNPs were synthesized using C. colocynthis fruit and its characterization was done by UV-visible spectroscopy, TEM, XRD and FT-IR. The 90% ethanol was used for extract preparation. Antioxidant activity was analyzed by DPPH and the Hydrogen Peroxide (H2O2) method. In vitro anti-arthritic activity was tested by xanthine oxidase inhibition, protein denaturation and HRBC membrane stabilization assay.
Results: The synthesized CC-AgNPs were confirmed by UV-vis spectroscopy and TEM images displayed spherical shapes with 10-45 nm size range. Furthermore, the functional groups and crystalline structure of CC-AgNPs were determined by FT-IR and XRD analysis. The biosynthesized CC-AgNPs exhibited an excellent free radical scavenging ability than EFECC. In anti-arthritic activity, the CC-AgNPs showed effective inhibition of xanthine oxidase production, protein denaturation, and damaged RBC membranes compared to EFECC.
Conclusion: The antioxidant activities and in vitro anti-arthritic assays revealed that CC-AgNPs are better anti-gout agents than EFECC. This research suggested that biosynthesized silver nanoparticles from C. colocynthis fruit are an important target in the field of anti-gout drug discovery.
Introduction :
Gout is a dangerous disease affecting people all over the globe and is induced by an accumulation of uric acid in the blood. This causes aggressive rheumatism and extreme pain by triggering the synthesis and deposition of Monosodium Urate crystals (MSU) in the joints 1. Increased secretion of uric acid and high conception of a purine-rich diet develop hyperuricemia. Gout and hyperuricemia are caused by the oxidation of xanthine and hypoxanthine into uric acid, which is catalyzed by the xanthine oxidase enzyme. High accumulation of urate crystals increased the MSU level in blood due to poor glomerular filtration 2. The increased MSU crystals were recently recognized in inflammatory and degenerative tissue reactions involving gouty arthritis through TLR2 and TLR4 receptors 3. The purpose of rheumatoid arthritis treatment is to relieve symptoms, delay the progression of the disease, and enhance the quality of life for patients. Currently, Disease-Modifying Antirheumatic Medicines (DMARDs) or Non-Steroidal Anti-Inflammatory Medications (NSAIDs) are being replaced by innovative biological agents such as TNF monoclonal antibodies in the treatment of arthritis 4. The above therapeutic medications minimize inflammation, joint pains and bone destruction, but their long-term side effects are unclear. Gastric ulcers, hematologic toxicity, hepatic fibrosis and immunological responses are some of the long-term risks of medications 5.
Herbal products have been used to cure a lot of diseases since ancient times. Nowadays researchers focused on developing therapeutic drug compounds from plant sources because currently available medicines either have negative impacts or are extremely expensive. Nature has provided us with a wide variety of herbal plants that are abundantly spread around the world as a source of medicinal substances for the prevention and treatment of a variety of ailments 6. Many of the important scientific innovations of 21st-century, research and development are believed to be based on nanotechnology. The production of nanoparticles from plant extracts could be used as both reducing and stabilizing agents 7. The plant extract composition triggers the nanoparticles' functionality. Silver particles were synthesized using multiple approaches, including chemical and physical processes, photochemical reduction, heat evaporation and electrochemical reduction 8. The dimensions of nanoparticles typically range from 1 to 100 nanometers and are an active field of research in nanoscience and nanotechnology 9. Pharmaceutical industries are currently focusing on developing green mediated nanoparticles with various biomedical applications to reduce toxicity and side effects. Silver nanoparticles (AgNPs) are particularly found in skin ointments and lotions for burns and open wounds 10.
Citrullus colocynthis L. (C. colocynthis), which belongs to the Cucurbitaceae family and is generally known as "Colocynth," is found in desert regions of the world 11. This plant is native to the Mediterranean region and Asia, however, it can be found in deserts throughout Asia, Europe, and North Africa. The fruits are utilized in Indian traditional medicine to treat a variety of ailments, and people use them to treat joint inflammation, rheumatism, and joint pain 12. According to a literature survey, C. colocynthis also has anti-inflammatory, antioxidant, anti-diabetic, antibacterial and anti-cancer properties 13. The alkaloidal fraction of the fruit extract inhibited the growth of breast and liver cancer cells, providing hope for anticancer potential 14. Therefore, the present comparative study was performed to investigate the antioxidant and anti-gout arthritic effect of synthesized silver nanoparticles from C. colocynthis Fruit (CC-AgNPs) and ethanol extract (EFECC).
Materials and Methods :
Chemicals used: The chemicals used in this research work such as Ethanol, Bovine Serum Albumin (BSA), Acetyl Salicylic acid and Allopurinol were purchased from SD Fine Chemicals Ltd. DPPH, Hydrogen Peroxide (H2O2), Phosphate Buffered Saline (PBS) (pH=7.4), Methanol, and Xanthine oxidase were obtained from Sigma Aldrich.
Collection and extraction of C. colocynthis fruit: The C. colocynthis fruits were collected from the local region of Madurai, Tamilnadu, India and its identification was confirmed by the Botanical Survey of India (BSI), Coimbatore, India. Collected fruit samples were washed with distilled water to remove impurities and dried in shade. The shade-dried C. colocynthis fruits (500 g) were ground to a fine powder. The fruit extract was prepared using 300 ml of 90% ethanol, added to 100 g of fruit powder and incubated at 37°C for 48 hr in a shaker incubator. The extracts were filtered with three different filters of mesh cloth, filter paper and Whatman no. 1 filter paper and evaporated. This process yielded an ethanolic extract of fruit C. colocynthis (EFECC). It was stored in a deep freezer for further analysis.
Synthesis of silver nanoparticles from C. colocynthis fruit: 1 g of fruit powder was weighed and boiled with 100 ml of distilled water at 60°C for 5 min. After boiling the extract was filtered through Whatman no. 1 filter paper. Then the filtrate was used for silver nanoparticle synthesis. To prepare silver nanoparticles, 10 ml of fruit extract was added into 90 ml of 1 mM silver nitrate solution in a conical flask and was kept in a mechanical shaker. The reduction was started at 1 hr at room temperature, resulting in color changing of aqueous silver nitrate solution indicating the formation of AgNPs. The bio-reduction of silver ions was monitored periodically by measuring UV vis spectra at 250-750 nm 15.
Characterization of nanoparticles: The biosynthesized CC-AgNPs were initially identified visually and then confirmed by double-beam UV-visible spectroscopy (Perkin-Elmer MA, USA). The structural characterization was confirmed by Transmission Electron Microscope (TEM). The crystalline nature of CC-AgNPs was examined using X-ray diffraction (Bruker AXS, Inc., Madison, USA). The bioactive groups in the fruit extract mediated AgNPs were evaluated by Fourier Transform Infrared spectroscopy (FTIR), Perkin-Elmer, MA, USA.
In-vitro antioxidant activity: DPPH radical scavenging assay: In the DPPH free radical scavenging assay, different concentrations (100, 200, 300, 400, and 500 µg/ml) of the test samples (EFECC and CC-AgNPs) were mixed with 1.0 ml of DPPH solution (0.1 mM) in ethanol. Similarly, ascorbic acid was utilized as a known standard, and the results were measured spectrophotometrically at 517 nm after 30 min of incubation 16.
H2O2 radical scavenging assay: In this assay, H2O2 (40 mM) was prepared in pH=7.4 PBS. Various concentration ranges (100-500 µg/ml) of the test samples (EFECC, CC-AgNPs and ascorbic acid) were separately added to H2O2 solution (0.6 ml). The reaction mixture was incubated for 10 min and observed spectrophotometrically at 230 nm 16.
In vitro anti-arthritic activity
Xanthine oxidase inhibitory assay: The xanthin oxidase inhibitory activity of EFECC and CC-AgNPs was assessed by the method of Rifaath 17. In brief, 1 ml of test samples (100-500 µg/ml), 2.9 ml of pH=7.4 PBS and 0.1 ml of xanthine oxidase (0.1 U/ml) were prepared for analysis. The reaction mixture was incubated at 25°C for 10 min and then a substrate solution of 150 M xanthine was added. The reaction was then inhibited with 1 ml of hydrochloric acid (1 N), and the absorbance was measured by a UV spectrophotometer at 290 nm. As a positive control, allopurinol was used. The xanthine oxidase activity was expressed in percentage inhibition calculated by the following formula:
% Inhibition = (1-As/Ac)´100
Whereas, As–Absorbance of test sample, and Ac- absorbance of control.
Protein denaturation study; The effect of EFECC and CC-AgNPs on protein denaturation assay was determined for anti-inflammatory potential and the method was followed by Chaithanya et al 18. Different concentrations of EFECC and CC-AgNPs (100-500 µg/ml) were added to 2 ml of 1% BSA and incubated at room temperature for 10 min. Later the mixture was heated in a water bath at 50°C for another 20 min. The inhibition level was measured spectrophotometrically at 660 nm.
HRB membrane stabilization test: In vitro anti-inflammatory activity was determined using the HRBC approach 19. Healthy volunteers' blood samples were drawn and treated with an appropriate amount of sterilized Alsevers solution. The packed cells were separated from the blood solution by centrifugation at 3000 rpm. Isosaline solution was used to wash the packed cells, and isosaline was used to make a 10% v/v cell suspension. The inhibitory activity was examined from this HRBC suspension. Different concentrations of test samples, standard acetylsalicylic acid were mixed individually with 1 ml of PBS, hyposaline (2 ml) and HRBC suspension (0.5 ml). All the above reaction mixtures were incubated at room temperature for 30 min before being centrifuged at 3000 rpm. The hemolysis level of supernatant liquid was determined spectrophotometrically at 560 nm.
Statistical analysis: The obtained results were expressed in Mean±SD. Microsoft Excel 2019 was used to compare the data of the control and test samples. If the p-value is <0.05, the results were considered statistically significant.
Results :
Synthesis and characterization of CC-AgNPs: The development of colloidal AgNPs from aqueous extract of C. colocynthis fruit when reacted with 1 mM silver nitrate (AgNO3) solution, slowly changed its color from pale yellow to deep brown color (Figure 1). After 48 hr of incubation, the AgNPs were completely reduced. The synthesized silver nanoparticles were abbreviated as CC-AgNPs. UV-visible spectroscopy is the most useful method for determining the reduction rate of biosynthesized silver nanoparticles. The UV spectra of CC-AgNPs were observed between 250-750 nm and found a strong SPR band at 450 nm (Figure 2), which confirmed the stability of CC-AgNPs. TEM analysis was used to examine the surface morphology of silver nanoparticles derived from C. colocynthis fruit extract. The TEM images demonstrated that synthesized CC-AgNPs were mostly spherical and some particles were round in shapes with 10-45 nm size (Figure 3). The crystalline structure of metal nanoparticles was examined using XRD analysis. Figure 4 demonstrated the XRD pattern of C. colocynthis fruit synthesized silver nanoparticles. The obtained peaks of CC-AgNPs were displayed at 2𝜃 values of 38.36°, 45.57°, 64.96° and 77.77° assigned to the Bragg’s reflection patterns of (111), (200), (220) and (311). According to this analysis, the biosynthesized CC-AgNPs have a Face-Cantered Cubic (FCC) structure. The observed peaks were well matched with standard silver structure (JCPDS file Number 84-0713). FTIR analysis was carried out to identify the possible functional groups in the C. colocynthis fruit extract-mediated AgNPs. Figure 5 represents the FTIR spectra of CC-AgNPs which have shown peaks at 3333.05 cm−1 and 1638.56 cm−1. The strong peak of CC-AgNPs was observed at 3333.05 cm−1 corresponding to -OH stretching of phenolic groups or N-H stretching of primary and secondary amines. The absorption band at 1638.56 cm−1 represents C=O stretching of amide or aldehyde or ketone groups. These characterization analyses confirmed the biosynthesized CC-AgNPs.
Antioxidants potential of EFECC and CC-AgNPs: The antioxidant activity was determined by DPPH and H2O2 radical scavenging assay by using ascorbic acid as standard. There are five different concentrations (100, 200, 300, 400 and 500 µg/ml) of EFECC, as well as CC-AgNPs which were used to access their free radical scavenging ability. DPPH is a stable synthetic free radical that is easily reduced by fruit extract and silver nanoparticles by absorbing or donating electrons, resulting in the development of the hydrazine molecule, which changes the color of DPPH from purple to yellow. The % inhibition of DPPH was determined by ascorbic acid, EFECC and CC-AgNPs. The free radical scavenging inhibition was gradually increased dose-dependently (Figure 6). This study showed that higher concentrations reported high scavenging ability for EFECC (72.33%), CC-AgNPs (79.9%) and standard ascorbic acid (82.16%). It has been shown that synthesized CC-AgNPs showed significantly higher DPPH radical scavenging ability than EFECC.
The H2O2 scavenging activity of EFECC and CC-AgNPs was compared to standard ascorbic acid. This investigation showed increasing scavenging ability with increasing concentration of EFECC and CC-AgNPs (Figure 7). The higher % inhibition was found at 500 µg/ml of EFECC (52.13%) and CC-AgNPs (59.2%). Finally, CC-AgNPs exhibit comparatively better free radical scavenging activity.
In vitro anti-arthritic activity: Xanthine oxidase inhibitory assay: In vitro anti-arthritic activity of EFECC, CC-AgNPs and standard allopurinol was assessed using xanthin oxidase inhibitory activity and the findings were expressed as % inhibition. According to the results of the xanthin oxidase investigation, the percentage inhibition of EFECC and CC-AgNPs showed increase dose-dependently and the higher inhibition was found to be 68.33% and 78.55% respectively at 500 µg/ml concentration (Figure 8). In the present study, CC-AgNPs showed better inhibition activity of xanthin oxidation when compared to EFECC.
Protein denaturation assay: The anti-inflammatory activity of EFECC and CC-AgNPs was measured by the inhibition of protein denaturation assay. In this determination, a better inhibition was observed at a higher concentration (500 µg/ml), which also showed concentration-dependent protein denaturation inhibition (Figure 9). Significantly higher inhibition was observed in CC-AgNPs (65.17%) compared to EFECC (53.87%), which were significantly nearer to standard acetylsalicylic acid.
HRBC membrane stabilization assay: The heat and hypotonic saline-induced damage to the RBC membrane was stabilized by EFECC and CC-AgNPs and were comparable with standard acetylsalicylic acid. The EFECC showed a maximum (54.33%) RBC membrane stabilization at 500 µg/ml, whereas the CC-AgNPs were found to be 62.3% and standard acetylsalicylic acid showed 63.06% at the maximum concentration (Figure 10). This study also revealed that synthesized CC-AgNPs effectively stabilized the RBC membrane than EFECC.
Discussion :
In our present investigation, we described an efficient and ecologically friendly approach for the development of silver nanoparticles using the aqueous fruit extract of C. colocynthis, which has excellent antioxidant and anti-arthritic properties. UV-visible spectrum detected SPR peak at 450 nm (Figure 2), TEM examination indicated spherical-shaped CC-AgNPs with 10-45 nm size range (Figure 3) and XRD confirmed CC-AgNPs' crystalline structure (Figure 4). FT-IR analysis confirmed the presence of phenolic group, primary and secondary metabolites in the CC-AgNPs (Figure 5). The results of in vitro antioxidant activity against DPPH and H2O2 radicals after reactions with CC-AgNPs showed better inhibitory action than EFECC (Figures 6 and 7). The improved free radical scavenging activity of green synthesized silver nanoparticles may be attributed to the interaction effect of AgNPs and the bioactive chemicals of plant extract that serve as a capping agent on nanoparticles’ surface 20. Similarly, AgNPs produced with extracts from Elephantopus scaber 21 and Coleus aromaticum 22 reported increased free radical scavenging activity.
The CC-AgNPs exhibited excellent anti-gouty arthritic activity through RBC membrane stabilization, inhibition of xanthine oxidase enzymes and protein denaturation. Gouty arthritis therapy aims to achieve a variety of requirements to reduce inflammation, stiffness and should block the excess secretion of uric acid 23. Nanomedicine has considerably improved the pharmacological and pharmacokinetic profiles of traditional drugs used to treat inflammatory arthritis 24,25. In the present investigation, the ethanolic fruit extract of C. colocynthis and CC-AgNPs effectively inhibited the protein denaturation enzyme and RBC membrane stability. Similarly, the Phyllum tomentosum leaves extract mediated silver nanoparticles showed strong anti-inflammatory activity through protein denaturation, RBC stabilization and xanthine oxidase analysis 26. Previous studies reported that the AgNPs from plant extract possessed excellent antiarthritic activity due to their small size and potential biological system interaction 27,28. However, inflammatory cells were able to internalize smaller nanoparticles more easily than their larger particles. The nanoparticles with <400 nm can effectively penetrate into the enlarged cells in rheumatoid arthritis 29,30. Compared to previous reports, the CC-AgNPs with 10-45 nm size ranges potentially inhibited the anti-inflammatory markers.
The current treatment for gout is xanthine oxidase inhibitors, which prevent the secretion of uric acid from purines. Allopurinol is the standard xanthine oxidase inhibitor, that is most commonly recommended to patients for gout treatment 31. On the other hand, there is a need to create naturally occurring molecules that have the potential to inhibit the xanthine oxidase enzyme to minimize the adverse effects of drugs 32. Thus, C. colocynthis fruit-mediated AgNPs and its ethanolic extract were investigated against inhibition of xanthine oxidase. This study showed better action of CC-AgNPs with 78.55% inhibitory effect which was significantly similar to standard allopurinol (Figure 8). This clearly demonstrates that the anti-arthritic effect comes from the combination of silver nanoparticles and the stabilizing phytoconstituents in C. colocynthis fruit 33. Studies by Mani A et al 34 have significantly found potent anti-arthritic activity of AgNPs synthesized from Piper nigrum extract. The nanoparticles drug delivery system indicated that the nanoparticles were capable of depositing into the affected joints and imposing excellent therapeutic efficacy with acceptable biosafety 33. According to the activity analysis, the CC-AgNPs have the ability to reduce inflammation and treat gout arthritis.
Conclusion :
The silver nanoparticles were synthesized from C. colocynthis fruit and were confirmed by various spectroscopic techniques. The CC-AgNPs were 10-45 nm in size with spherical shaped, crystalline in nature. The plant source-mediated nanoparticles are non-toxic and ecologically friendly due to the presence of bioactive molecules involved in the reduction process. The therapeutic effect of synthesized CC-AgNPs and ethanol extract of fruit were successfully investigated for antioxidant and anti-arthritic activity. The presence of phenols, proteins and amine groups in the fruit extract coated CC-AgNPs exhibited high DPPH and H2O2 radical scavenging activity than EFECC. In anti-arthritic assays, CC-AgNPs displayed better inhibition activity of xanthine oxidase enzyme and inflammatory markers than ethanol extract of the fruit. Thus, our findings suggested that biosynthesized CC-AgNPs have anti-gout potential and might play a key role in the development of novel and effective anti-gout therapies.
Acknowledgement :
We gratefully acknowledge Er. A.C.S. Arun Kumar, President, Dr. M.G.R Educational and Research Institute University for providing the necessary facilities.
Conflict of Interest :
The authors declare that they have no known competing financial interests in this paper.

Figure 1. Bio-reduction of Ag ions to AgNPs using Citrullus colocynthis fruit extract with 1 mM silver nitrate (A) Initial stage (B) complete reduction after 48 hr.
|
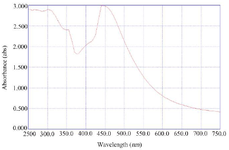
Figure 2. UV-Vis Spectroscopy analysis of CC-AgNPs colloidal solution observed a strong SPR band at 450 nm.
|
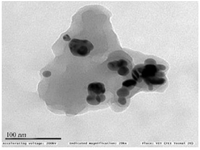
Figure 3. TEM analysis found a surface morphology of synthesized CC-AgNPs as round shaped with 10-45 nm size ranges.
|
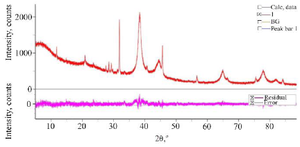
Figure 4. XRD analysis confirmed the crystalline structure of C. colocynthis fruit-mediated AgNPs.
|
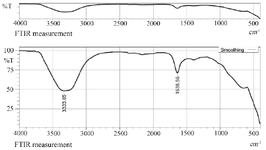
Figure 5. FTIR analysis identified the presence of biochemical components in the fruit extract involved in the CC-AgNPs synthesis mechanism.
|
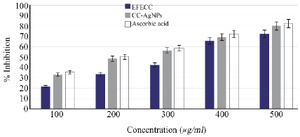
Figure 6. DPPH free radical scavenging potential was observed to be EFECC >CC-AgNPs> ascorbic acid and the %inhibition was increased dose-dependently.
|
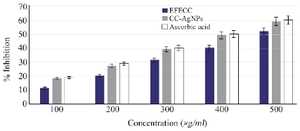
Figure 7. Hydrogen peroxide scavenging activity was observed in EFECC >CC-AgNPs> ascorbic acid and H2O2 inhibition also increased dose-dependently.
|
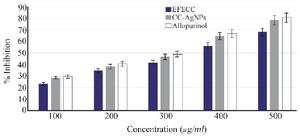
Figure 8. Xanthine oxidase inhibitory analysis proved CC-AgNPs effectively inhibit the xanthin oxidase production similar to Allopurinol standard drug.
|
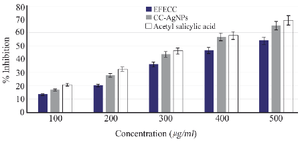
Figure 9. The protein denaturation assay observed the anti-inflammatory activity of Acetyl Salicylic acid >CC-AgNPs> EFECC.
|
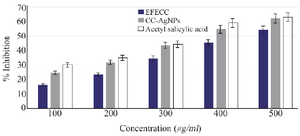
Figure 10. RBC membrane stabilization analysis demonstrated that CC-AgNPs effectively inhibit the damaged RBC membrane when compared to EFECC.
|
|