Isolation, Molecular Identification and Antibacterial Potential of Marine Bacteria from Deep Atlantic Ocean of Morocco
-
Chbel, Asmaa
-
Laboratory of Physiopathology, Molecular Genetics & Biotechnology, Faculty of Sciences Ain Chock, Research Center of Health & Biotechnology, Hassan II University of Casablanca, 20100 Casablanca, Morocco
-
Rodriguez-Castro, Jorge
-
Laboratory of Molecular Systematics, Department of Biochemistry & Molecular Biology, CIQUS University of Santiago de Compostela, Santiago, Spain
-
Quinteiro, Javier
-
Laboratory of Molecular Systematics, Department of Biochemistry & Molecular Biology, CIQUS University of Santiago de Compostela, Santiago, Spain
-
Rey-Méndez, Manuel
-
Laboratory of Molecular Systematics, Department of Biochemistry & Molecular Biology, CIQUS University of Santiago de Compostela, Santiago, Spain
-
Serrano Delgado, Aurelio
-
Institute of Plant Biochemistry and Photosynthesis (IBVF), CSIC-University of Sevilla, 41092 Seville, Spain
-
Soukri, Abdelaziz
-
Laboratory of Physiopathology, Molecular Genetics & Biotechnology, Faculty of Sciences Ain Chock, Research Center of Health & Biotechnology, Hassan II University of Casablanca, 20100 Casablanca, Morocco
-
El Khalfi, Bouchra
-
Laboratory of Physiopathology, Molecular Genetics & Biotechnology, Faculty of Sciences Ain Chock, Research Center of Health & Biotechnology, Hassan II University of Casablanca, 20100 Casablanca, Morocco
Abstract: Background: Antibiotic resistance is an important concern for the public health authorities at global level. It is detrimental to human and environmental ecosystems, thus, there is a big need for natural bioactive compounds. In this work, we aimed to find out biomolecules derived from marine bacteria that may constitute an alternative to antibiotics.
Methods: We isolated and identified thirty one marine bacteria collected from deep ocean water in central coast of Safi city, Morocco. Then, we induced biomolecules production in six marine bacterial strains. The extracts were tested for their antibacterial activity against gram-negative and gram-positive bacteria such as Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 33592 and Listeria monocytogenes ATCC 19117. Furthermore, we partially analyzed the chemical composition of these biomolecules and evaluated their sensibility to different temperatures.
Results: The six marine bacteria were able to produce molecules which inhibited the three pathogenic strains with high inhibition zones reaching 27 mm. These molecules were characterized by heat stability from 60 to 121°C relying on each strain.
Conclusion: The produced molecules may offer a great potential to pharmaceutical industries as they may constitute an alternative to antibiotics that are becoming less effective due to the emergence of drugs resistance.
Introduction :
The marine environment is the largest aquatic ecosystem on Earth hosting a huge ecological, chemical and biological diversity. Sea water rich of halogens such as chlorine, bromine, iodine, and sulphate provides the largest living space for numerous species of macro- and micro-organisms surviving in a hostile environment in terms of extreme temperatures, and notable changes in salinity and pressure 1.
During the last few decades, many studies have focused on the bioactive compounds produced by micro-organisms, especially marine bacteria 2. Adapted to oligotrophy, marine bacteria are distinguished from those adapted to nutrient rich environments by genetic and metabolic characteristics. They can adjust their enzymatic mechanisms and produce a great variety of molecules representing new bioactive compounds 3, such as antimicrobial, antioxidant and antitumor molecules with a great pharmaceutical potential 4-6. Thus, they are also used in industrial fields as stabilizers or fish preservatives 7.
Marine bacteria are also recognized to produce extracellular polymers often in the form of exopolysaccharides 8. Their production is often correlated to the establishment of the biofilm growth mode, during which important matrix components are formed. Their functions include adhesion and colonization of surfaces, protection of bacterial cells and support for biochemical interactions between bacteria and the surrounding environment 1. Most of exopolymers consist of polysaccharides and proteins and they may be homopolymeric or heteropolymeric in configuration. These compounds have a great role in the maintenance of marine environments and the preservation of this ecosystem by different processes among which sedimentation, particle formation, cycling of dissolved metals and dissolved organic carbon 9,10.
Furthermore, marine bacteria appear to be a promising source of novel bioactive natural products for drugs development necessary for the decrease of infectious diseases caused by the propagation of drug-resistant pathogens classified as a threat to human health, by the World Health Organization (WHO) 7,8,11,12. For this reason there is a renewed interest in exploring the marine environment and its potential bioactive compounds for novel antimicrobial agents.
In the present work, we isolated and identified thirty one bacterial strains from the deep Atlantic ocean seawater of Safi city, Morocco. Then, we evaluated their capacity to produce biomolecules using different culture conditions. Afterward, we tested the antibacterial activity of the produced molecules against three bacterial strains most commonly found in human pathologies. Finally, we partially analyzed the chemical composition of these bioactive molecules and evaluated their heat stability to provide future potential application in different industrial fields.
Materials and Methods :
Isolation of bacterial strains
Water samples were collected into sterile glass bottles from two sites in the deep Atlantic ocean of Safi city in Morocco (Figure 1) down to 914.4 and 1.8288 meters depth, with a temperature of 14-18°C. We simply lower the bottles to the samples depth where the coordinates of each site were as follows: Site 1: N 31°51’559’’/ W 10°24’599’’, Site 2: N 31°35’973’’/ W 10°26’183’’. Then, the water samples were taken to the laboratory for study. We filtered 500 ml with a 0.45 μm millipore filter and a vacuum pump, and the filter was placed on a Petri dish containing Marine Agar medium (MA) (Difco 2216). The incubation was carried out at 25°C for 24 hr, in which the bacteria had an optimum growth. Then, colonies were picked according to their color and form and were streaked onto MA Petri dishes.
Bacterial identification
Gram staining: Gram staining method was carried out using bacteriological standard procedures 13.
Genomic DNA extraction
A phenol-chloroform method was used for the extraction of genomic DNA from each bacterium 14. Five ml of bacterial suspension were transferred to sterile microcentrifuge tubes and spun 10 min at 5000 g, followed by the addition of 467 µl TE buffer (Sigma-Aldrich), 30 µl of 10% (w/v) SDS (Sigma-Aldrich) and 3 µl of proteinase K (20 mg/ml, Sigma-Aldrich). Samples were briefly mixed and then incubated for 45 min at 37°C and 15 min at 42°C. An equal volume of phenol-chloroform-isoamyl alcohol (25:24:1) mixture (Sigma-Aldrich) was added, carefully mixed and spun for 2 min. Then, the upper aqueous phase was transferred to a new tube and DNA was precipitated by adding 1/10 volume of 0.1 M sodium acetate and 0.6 volumes of isopropanol. After gently mixing until DNA precipitation, the slimy white material was spooled onto a glass rod and then washed with 70% (v/v) ethanol (Sigma-Aldrich), air dried and finally resuspended in 100-200 µl TE buffer. Concentrations of purified products were determined using a NanoDrop® ND-1000 spectrophotometer.
Amplification and sequencing of bacterial 16S rDNA
The region of the 16S ribosomal gene of the genomic DNA extracted from each bacterial strain was amplified by Polymerase Chain Reaction (PCR). The amplification of purified genomic DNA fragment (10 ng per reaction) was carried out with the following universal primer pair:
Forward 5’-AGAGTTTGATCCTGGCTCAG-3’ and Reverse 5’-ACGGCTACCTTGTTACGACTT-3’. The reaction mixture contained 3 μl of the Green GoTaq® Reaction Buffer, 0.8 mM of each deoxynucleotide triphosphate, 80 nM of each primer, 0.5 mM MgCl2, and 2.5 units of GoTaq® DNA Polymerase (Promega).
Amplification was performed in a DNA thermal cycler that consisted of an initial denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 94°C for 40 s and annealing at 55°C for 40 s, extension at 72°C for 1 min and a final extension at 72°C for 10 min. The PCR products were separated in a 1% (w/v) agarose gel and detected by staining with ethidium bromide (1 µg/ml).
Sequencing was performed using Big Dye Terminator version 1.0 kit (Applied Biosystems, Spain), sequences were then aligned with SeaView v.4 software 15 following a Neighbor-Joining approach (CLUSTAL O) to generate a bootstrapped distance phylogenetic tree.
Biomolecules production
Six marine bacterial strains of different genera were selected for these study tests. They were cultured in Marine Broth at 25°C during 48 hr within different culture conditions, as shown in table 1, in order to induce them and evaluate their capacity to produce biomolecules.
A culture centrifugation was carried out at 10000 g at 4°C for 10 min to obtain supernatants containing biomolecules. Then, antibacterial activity was performed using:
- Supernatant directly.
- Filtered supernatant with 0.2 µm filter, in order to
eliminate bacterial cell debris.
- Precipitated supernatant, obtained by adding two volumes of 95% cold ethanol to supernatant which was then stored overnight at 4°C to allow precipitation of exopolysaccharides. The mixture was centrifuged at 5000 rpm for 20 min at 4°C to collect the polysaccharides, then, the pellet was dried to evaporate the remaining ethanol and was dissolved in milliQ water 16.
Antibacterial activity assay
To assay the inhibitory activity of marine biomolecules and to study their physico-chemical properties, the agar-well diffusion method was employed according to 17 with some modifications. Escherichia coli ATCC 25922 (E. coli), Staphylococcus aureus ATCC 33592 (S. aureus) and Listeria monocytogenes ATCC 19117 (L. monocytogenes) were used as indicator
strains in assays of antibacterial activity. They were isolated in the Laboratory of Physiopathology, Molecular Genetics and Biotechnology from Hospital settings and were cultured in 5 ml of Luria-Bertani broth (LB) at 37°C for 24 hr. Then, 20 ml of LB agar medium (0.7% w/v) was inoculated with 10⁶ CFU/ml of indicator strains fresh culture and were poured into a Petri dish. Four wells of 5 mm diameter were performed into inoculated LB agar medium of each Petri dish and filled with 50 µl of:
- Well 1: Supernatant directly.
- Well 2: Filtered supernatant.
- Well 3: Precipitated supernatant.
- Well 4: Rifampicin (5 mg/ml) used as positive control.
The plates were incubated at 37°C, and the diameters of the bacterial growth inhibition were measured.
Effect of temperature and proteinase K on marine biomolecules stability
To determine the heat stability of bacterial biomolecules, each culture supernatant was heated to 60°C for 60 min, 80°C for 30 min, 100°C for 20 min, and 121°C for 15 min, then they were treated with proteinase K and tested for antibacterial activity 17.
Carbohydrates and protein analysis
Phenol-sulfuric acid reaction was used for total carbohydrates analysis according to Dubois M 18. Two hundred μl of sample was mixed with 200 μl of phenol 5%. One ml of sulfuric acid 95% was added. After 10 min, the solution was vortexed. An orange color was formed after 30 min. Then, the absorbance was measured at 490 nm. Furthermore, protein quantification was carried out with the Bradford method 19.
Statistical analysis
The results of antibacterial activity assay were given as means±standard deviation (SD). Then, the data were analyzed using Prism 8.0 software using two way ANOVA followed by Tukey’s multiple comparisons test where a statistical significance difference was shown when p<0.05.
Results :
Bacterial identification
The nucleotidic sequences of 16S rDNA gene were analyzed in order to reveal the identities of bacteria isolated from deep ocean water in the coastal region of Safi city in Morocco using NCBI Blast. The results have shown a great variety of strains in the two collecting sites as represented in the phylogenetic trees (Figures 2 and 3). Gram-positive and gram-negative species were present, belonging to Proteobacteria and Firmicutes phyla. The taxonomic lineage of Alteromonas, Idiomarina, Pseudoalteromonas, Cobetia, and Vibrio species is related respectively to Proteobacteria, Gammaproteobacteria, Alteromonadales, Alteromonadaceae/Idiomarinaceae/Pseudoalteromonadaceae/Oc-eanospirillales, Halomonadaceae/Vibrionales, Vibrionaceae, while Bacillus, Planococcus, and Staphylococcus species belong respectively to Firmicutes, Bacilli, Bacillales, Bacillaceae/Planococcaceae/Staphylococc-aceae.
Antibacterial activity
Six bacteria of different genera were selected for the antibacterial activity assay: Alteromonas hispanica B 35 (OL757740), Vibrio sp. JT-FAJ-16 (OL757745), Pseudoalteromonas sp. AECF-16 (OL757734), Cobetia marina strain A7 (OL757736), Staphylococcus haemolyticus strain Sq21 (OL757738) and Planococcus maritimus strain Xmb052 (OL757737). They secreted molecules within all culture conditions used for bacterial induction (Tables 2-5). Following each exposure, supernatants enhanced antimicrobial activity towards the three pathogenic bacteria: Escherichia coli (E. coli), Staphylococcus aureus (S. aureus) and Listeria monocytogenes (L. monocytogenes). The bacterial exposure to UV light for 1 min remains the most effective induction method used in this work. As shown in table 2, the growth of E. coli, L. monocytogenes and S. aureus was strongly inhibited by the culture supernatant molecules released by Vibrio JT-FAJ-16 followed by Pseudoalteromonas sp. AECF-16, where the diameters of inhibition were ranging from 19.3 to 27.0 mm. Additionally, filtered supernatants showed no difference in inhibition diameters in comparison with supernatants that were used directly. In comparison with the results obtained with rifampicin which inhibited the three pathogenic bacteria (E. coli: 25 mm, S. aureus: 22 mm and L. monocytogenes: 20 mm), we can report that marine bacterial molecules released by Vibrio sp. JT-FAJ-16, Pseudoalteromonas sp. AECF-16, Cobetia marina strain A7 and Staphylococcus haemolyticus strain Sq21 showed larger inhibition diameters. However, there was a significant difference (p<0.05) between the antibiotic, Planococcus maritimus strain Xmb052 and Alteromonas hispanica B 35 supernatants (data not shown). Moreover, it is important to note that active biomolecules of the precipitated supernatants gave good inhibition diameters reaching 27.2 mm. Hence, according to the statistical analysis, there was a significant differ-ence (p<0.05) between the antibiotic and Planococcus maritimus strain Xmb052, Cobetia marina strain A7 and Alteromonas hispanica B 35 biomolecules, with no difference with Vibrio JT-FAJ-16, Staphylococcus haemolyticus strain Sq21 and Pseudoalteromonas sp. AECF-16 (data not shown).
Heat, enzyme stability and composition analysis of the secreted biomolecules
To characterize the chemical nature of the inhibitory substances produced by the selected marine strains, a study of the stability of active substance samples was carried out in different heat temperatures and in the presence of a proteolytic enzyme (proteinase K). Our results show that the molecules activity was not affected by heat at 121°C for Staphylococcus haemolyticus strain Sq21. The same stability was shown for Pseudoalteromonas sp. AECF-16, Cobetia marina strain A7 and Alteromonas hispanica B 35 to a temperature up to 100°C, while molecules released by Planococcus maritimus strain Xmb052 and Vibrio JT-FAJ-16 could withstand a temperature of 80°C. In addition, molecules activities were not lost after treatment with proteinase K which indicates the non proteinaceous nature of the active substances (Table 6). Furthermore, the composition of the secreted molecules with carbohydrates and protein nature was quantified. Results show that active biomolecules released by the six marine bacteria were mainly composed by carbohydrates with a low quantity of proteins (Figures 4 and 5).
Discussion :
In the marine environment, most of bacteria are gram-negative; this cell wall was reported to be better adapted for survival of bacteria in the marine environment because of its structural conformation and com-position 20. Bacteria can thrive in harsh oceanic conditions particularly because of the anionic O-antigens composing a part of lipopolysaccharide which form the major component of the outer membrane of gram-negative bacteria 20. The marine environment is characterized by a taxonomic richness and the partition of bacterial communities is dependent on geographical locations. The isolated bacteria belong to different phyla among which Proteobacteria, known by the high heterogeneity displayed by the bacteria belonging to this phylum. A common trait of Proteobacteria is the gram-negative staining and, thus, the presence of the lipopolysaccharide as a major component in the outer membrane. Proteobacteria represent the largest phylum within the bacteria domain. It is divided into six classes Gammaproteobacteria, Alphaproteobacteria, Deltaproteobacteria, Betaproteobacteria, Epsilonproteobacteria and Zetaproteobacteria. Then, comes the second phyla: Firmicutes most of which bacteria have a gram-positive cell wall structure. Species belonging to this phylum are affiliated with three classes Bacilli, Clostridia, and Erysipelotrichia 21.
Marine bacteria are uniquely important to life. Their metabolic capacities allow them to produce bioactive compounds to prevent biofouling or to ward off predators in the marine environment 22. The novelty and diversity of marine molecules can be put to work in a number of biotechnology applications due to their various biological activities including anti-inflammatory, antiviral, antitumoral, antifungal and antifouling properties 23,24. In this work, we evaluated the ability of six marine bacteria to produce molecules that may inhibit drug-resistant bacteria. As known, the antibiotic resistance has become an urgent concern because of the possible horizontal gene transfer between bacteria which make antibiotics less effective and, consequently, infections become more complex to heal. Notably, the six marine species have produced molecules within the supernatant after being induced. This correlates with previous researches reporting that induction of marine bacteria by cross culture with a terrestrial bacterium or by addition of a mineral salt such as sodium chloride or a carbon source like glucose in the bacterial culture broth lead to production of antimicrobial compounds 25-27.
Moreover, it was reported that the exposure of marine bacteria to UV caused a stress to bacteria living in deep ocean and consequently lead to production of molecules 24,28,29. The supernatants enhanced an antimicrobial activity towards the three bacteria E. coli, S. aureus and L. monocytogenes. These results correlate with a previous study where several marine species within Pseudoalteromonas and Vibrio genera produced antibacterial compounds against S. aureus 30. Another study reported the ability of Vibrio species to produce molecules with attractive biological properties which showed antibacterial activities against S. aureus and E. coli besides other pathogens showing a potential use as antibiotics in pharmaceutical industries 24.
On the other hand, the produced molecules could withstand high temperatures and maintain their antibacterial effect even after being exposed to proteinase K. This correlates with the inhibition of bacterial pathogens by the precipitated supernatants with 95% ethanol which confirm the glucidic nature of the active molecules that may be polysaccharides secreted by bacteria outside their cells. Some previous researches reported a structural diversity of marine exopolysaccharides providing diverse biological properties among which the antibacterial activity against different pathogens. They were mostly heteropolysaccharides composed of different monomers, including neutral monosaccharides such as glucose, galactose, mannose and fucose, besides uronic acids as glucuronic acid and galacturonic acid 31-33. Hence, our biomolecules properties indicate that they may be useful in food industrial field as biopreservatives as well as the pharmaceutical industry, so as they may constitute an alternative to antibiotics that have become increasingly ineffective. This is in agreement with several studies highlighting the potential of using marine exopolysaccharides from bacteria belonging to different genera as Pseudoalteromonas, Vibrio, Pseudomonas, Halomonas, Alteromo-nas and Alcaligenes for biotechnological applications in the field of food, medical imaging, nano-drugs, bioremediation, cancer, anti-bacterial, tissue engineering, etc. 34-36. They have successfully inhibited the bacterial growth of a plethora of human pathogenic bacteria belongingto Acinetobacter, Bacillus, Campylobacter, En-terobacter, Enterococcus, Escherichia, Proteus, Pseudomonas, Salmonella, Streptococcus and Staphylococcus genera with more susceptibility of gram-negative bacteria than the gram-positive ones 37,38.
Acknowledgement :
We acknowledge the laboratory of Molecular Systematics of the University Santiago de Compostela for facilitating the sequencing of bacterial species.
Conflict of Interest :
The authors have no conflict of interest to declare.
Financial support. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
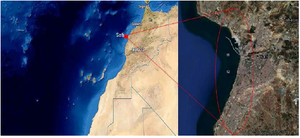
Figure 1. Location of the sampling area in deep Atlantic ocean of Morocco.
|
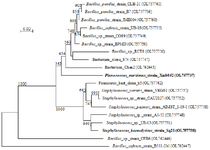
Figure 2. Phylogenetic tree of gram-positive marine bacterial isolates reported in this work based on 16S rDNA. Marine bacterial strains that were used in the next experiments are shown in bold. Scale bar represents nucleotide substitutions per sequence site
|
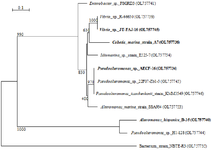
Figure 3. Phylogenetic tree of gram-negative marine bacterial isolates reported in this work based on 16S rDNA. Marine bacterial strains that were used in the next experiments are shown in bold. Scale bar represents nucleotide substitutions per sequence site.
|
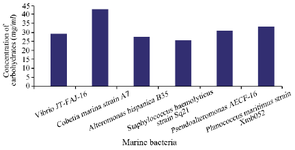
Figure 4. Quantitative carbohydrates analysis of biomolecules secreted by the six marine bacteria.
|
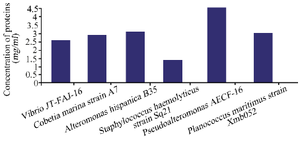
Figure 5. Quantitative proteins analysis of biomolecules secreted by the six marine bacteria.
|
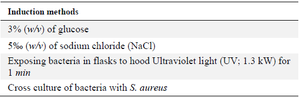
Table 1. Different methods of bacterial induction
|
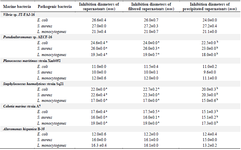
Table 2. Results of the antibacterial activity test using UV induction
Values are represented as mean ± SD of triplicate. Values in the same row with different superscript letters differ significantly (p<0.05).
|
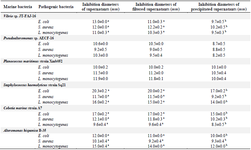
Table 3. Results of the antibacterial activity test using glucose induction
Values are represented as mean ± SD of triplicate. Values in the same row with different superscript letters differ significantly (p<0.05).
|
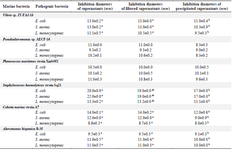
Table 4. Results of the antibacterial activity test using NaCl induction
Values are represented as mean ± SD of triplicate. Values in the same row with different superscript letters differ significantly (p<0.05)
|
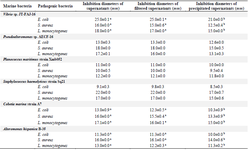
Table 5. Results of the antibacterial activity test using cross culture induction
Values are represented as mean ± SD of triplicate. Values in the same row with different superscript letters differ significantly (p<0.05).
|
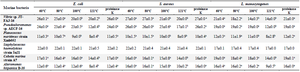
Table 6. Results of the antibacterial activity of biomolecules produced by the six marine bacteria after heat and proteinase K treatments
Values are represented as mean ± SD of triplicate. Values in the same row with different superscript letters differ significantly (p<0.05).
|
|