Optimization of Expression and Purification of Recombinant Mouse plac1
-
Rahdan, Shaghayegh
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Razavi, Seyed Alireza
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Nazari, Mahboobeh
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Shojaeian, Sorour
-
Department of Biochemistry, School of Medical Sciences, Alborz University of Medical Sciences, Karaj, Iran
-
Shokri, Fazel
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Amiri, Mohammad Mehdi
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Ramezani, Amin
-
Institute for Cancer Research, Shiraz University of Medical Sciences, Shiraz, Iran
-
 Zarnani, Amir-Hassan
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 42933031; E-mail: zarnania@tums.ac.ir, zarnania@gmail.com
Zarnani, Amir-Hassan
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 42933031; E-mail: zarnania@tums.ac.ir, zarnania@gmail.com
Abstract: Background: Placenta-specific 1 (PLAC1) is one of the recently-discovered Cancer-Testis-Placenta (CTP) antigen with restricted normal tissue and ectopic expression in a wide range of cancer cells from different histological origins. The production of recombinant human PLAC1 has already been optimized; however, no study has been reported so far on the production and purification of mouse plac1. In this study, mouse plac1 expression and purification was optimized in a prokaryotic system and the effects of the generated proteins on inducing humoral responses in mice were investigated.
Methods: A fusion protein containing full extracellular domain of mouse plac1, immunostimulatory peptides, tetanus toxin P2P30 and PADRE and KDEL3 signal (main plac1), and the same fragment without immunostimulatory peptides (control plac1) was produced. To optimize production and purification steps, different parameters including bacterial strain, cultivation temperature, cultivation time, IPTG concentration, culture medium, and also different buffers for purification of the recombinant proteins were tested. After confirming the identity of recombinant plac1 proteins with Western Blotting (WB) and ELISA assays, these proteins were subcutaneously injected in mice with Freund's adjuvant and the anti-plac1 antibody response was detected by ELISA.
Results: The optimal expression level of main and control plac1 was obtained in BL21 (DE3) and TB culture medium in the presence of 0.25 mM IPTG after 24 hr of induction at 15°C. The buffer containing 2% sarkosyl produced higher yield and purity. Our results showed specific reactivity of anti-human recombinant plac1 polyclonal antibody with both main and control plac1 recombinant proteins in WB and ELISA analysis. Both proteins induced humoral responses in mice; however, anti-plac1 antibody titer was significantly higher in sera of mice immunized with main compared to control plac1.
Conclusion: In this study, an optimized protocol for production and purification of mouse plac1 was reported and it was shown that insertion of immunostimulatory peptides in gene construct could efficiently enhance humoral immune responses against mouse plac1, which could potentially augment cellular immune responses against plac1 leading to more effective anti-cancer responses.
Introduction :
As an X-linked gene, placenta-specific 1 (PLAC1) encodes a membrane-associated protein which is mainly involved in placental development 1. The human PLAC1 and mouse plac1 gene maps 65 kb telomere to the HPRT gene at Xq26 2. Human PLAC1 consists of a large extracellular domain spanning amino acids 23 to 212 and a short transmembrane helix (amino acids 5 to 22), whereas mouse plac1 encodes a putative protein of 173 amino acids. A part of extracellular domain of PLAC1 consisting of amino acids 29 to 119 is homologous to the N-terminal sub-domain of the Zona Pellucida glycoprotein-3(ZP3) 3. At DNA and protein levels, mouse and human molecules show 75% and 60% identity, respectively 2.
PLAC1 has a restricted pattern of expression in normal cells confining primarily to the cells of the trophoblast lineage and testis 1,2, while it is ectopically expressed in a wide range of cancer cells including breast 3, endometrium 4, Non-Small Cell Lung Cancer (NSCLC) 5, hepatocellular carcinoma 6, colon 7, and gastric adenocarcinoma 8 and is involved in cancer progression. Recently, differential expression of human PLAC1 in prostate cancer was reported and a strong positive association between PLAC1 expression and Gleason score was shown 9. Subsequently, it was demonstrated that anti-PLAC1-SN38 conjugate exerted powerful and specific anti-cancer effects on human primary prostate cancer cells and prostate cell lines indicating potential usefulness of targeting PLAC1 for immunotherapy of patients with prostate cancer 10. In another study, melanoma cells expressed PLAC1 and time-and dose-dependent cytotoxic effects of drug-conjugated anti-PLAC1 antibody were observed in melanoma cancer cells 11. Based on its restricted expression in testis and placenta and also in a wide variety of cancers, PLAC1 was categorized as a Cancer-Testis-Placenta (CTP) antigen, a feature that PLAC1 shares with many other cancer testis antigens.
PLAC1 represents most features of an ideal target for cancer immunotherapy including limited expression in normal cells, availability to the antibodies based on its surface expression, high expression in cancer cells with different histologic origins, and its role in vital parameters of cancer cells including growth, proliferation, invasion, and survival 1.
Human PLAC1 expression was already optimized in prokaryotic systems producing PLAC1 with high purity and yield 12. Although human and mouse PLAC1 share common epitopes and may induce cross-reactive humoral immune responses, in vitro studies in murine cancer models need host-specific proteins. In this study, mouse plac1 expression and purification was optimized in a prokaryotic system. To this end, two recombinant plac1 proteins were produced and characterized including main plac1 as a fusion protein containing full extracellular domain of mouse plac1+tetanus toxin P2 and P30+PADRE+KDEL3 and a control plac1, the same fragment without P2P30 and PADRE peptides. P2 (QYIKANSKFIGITEL) and P30 (FNNFTVSFWLRVPKVSASHLE) peptides are immunogenic peptides in the first domain of tetanus toxin structure and PADRE (AKFVAAWTLKAAA) is a synthetic peptide that acts as a potent stimulator of helper T cells 13,14. KDEL3 signal has amino acid sequence KKDELRDELKDEL that enhances MHC class I and II presentation of antigen, leading to increases in the specific humoral and cellular responses 15. After confirming the identity of main and control plac1 recombinant proteins, their effects on inducing humoral responses in mice were investigated and compared.
Materials and Methods :
Construction of recombinant plac1 plasmid: Recombinant main plac1 construct (P2P30-PADRE-extracellular domain of mouse plac1 (Accession No: NM_019538)) was designed in our laboratory and synthesized in pBSK vector (Biomatik Company, Canada). In order to facilitate purification of recombinant protein with Ni-NTA column, a His6-tag sequence was designed at the C-terminal end of the construct (Figure 1). This construct was used as a template for subcloning the recombinant main plac1 into pColdi expression vector. At first, pBSK vector containing main plac1 was transformed into the competent TOP10 Escherchia coli (E. coli) (Novagen, USA). The transformed colonies were selected by culturing in LB agar plates containing ampicillin (100 μg/ml) and were confirmed by colony PCR. The colony PCR was run in 30 cycles using M13R and M13F specific primers (Table 1) and Taq DNA Polymerase 2x Master Mix RED (Ampliqon, Denmark). Each amplification reaction underwent initial denaturation at 95°C for 3 min followed by 30 cycles at 94°C for 30 s, 45°C for 1 min, 70°C for 1 min, and final extension was also performed at 70°C for 10 min. After colony selection, targeted colonies were cultured in LB broth medium containing ampicillin (100 μg/ml) and recombinant main plac1 miniprep was extracted using the Miniprep extraction kit (Favorgen, USA). Recombinant main plac1 miniprep was directly digested with NdeI and SalI restriction enzymes (Fermentas, USA) and ligated using T4 DNA ligase (Promega, USA) into pColdi expression vector (Promega, USA) digested with the same enzymes. The final miniprep was then transformed into the TOP10 E. coli competent cells and transformed colonies were selected as described above using pColdi universal primers in 25 cycles (Table 1). Plasmid in pColdi expression vector was extracted as mentioned above.
In order to produce the control plac1 miniprep (Figure 1), the final miniprep of the main plac1 was digested with HindIII (Fermentas, USA), self-ligated using T4 DNA ligase (Promega, USA), transformed into the TOP10 E. coli and final miniprep was extracted.
Recombinant plac1 expression and purification: Small scale expression of recombinant proteins: Expression of mouse plac1 was performed as described earlier 12. In brief, after confirming the sequence of the final minipreps by sequencing, they were transformed into the four E. coli strains (Novagen, USA), namely BL21 (DE3), Origami (DE3), Rosettagami (DE3) pLysS, and Shuffle T7 (DE3). For small scale expression, 5 ml LB or TB medium containing 100 μg/ml ampicillin for BL21 (DE3) and Shuffle T7 (DE3), 100 μg/ml ampicillin, 34 μg/ml chloramphenicol and 15 μg/ml kanamycin for Rosettagami (DE3) and 100 μg/ml ampicillin, and 15 μg/ml kanamycin and 15 μg/ml tetracycline for Origami (DE3) were inoculated with a fresh selected bacterial colony and grown overnight at 37°C. Next, 20 ml of fresh medium was inoculated with 400 µl overnight cultures and incubation was continued at 37°C with shaking (160 rpm) until the OD600 reached 0.5-1. After induction with 0.25 and 1 mM IPTG, further culturing at 15°C and 37°C for 3, 6, and 24 hr was performed followed by cell harvesting. Expression of the protein was assessed by SDS-PAGE. Among variables tested including expression host, cultivation temperature, cultivation time, concentration of IPTG, and culture medium, variables with optimal protein expression were selected for a large scale protein production.
For purification of main and control plac1, first expression of the proteins was tested in soluble fraction or inclusion body of the cell lysate. In this regard, different lysis buffers for inclusion body aggregates were screened and purification protocol was then optimized. To determine the optimal condition for solubilization of inclusion bodies and preparation of recombinant proteins, samples of both insoluble (precipitation) and soluble fractions (supernatant) were placed on 12% SDS-PAGE gels, and protein bands were visualized by Coomassie Brilliant Blue staining (BioRad, USA). Based on the results, buffer IIA and buffer D were selected for preparation and purification of main and control plac1. Composition of lysis buffers is listed in table 2. The methodology for cell lysate preparation using selected lysis buffers are summarized below.
For buffer IIA, bacterial cell pellet was resuspended first in lysis buffer I (50 ml lysis buffer per 100 ml of bacterial culture) (Table 2) for 1 hr on ice with internment vortexing followed by 12 rounds of 20s on/40s off sonication (Amplitude 70%). The cell lysate was then centrifuged at 13000 g for 30 min at 4°C and both insoluble and soluble fractions were collected. The insoluble fraction was lysed in lysis buffer IIA (50 ml lysis buffer per 100 ml of bacterial culture) (Table 2) for 1 hr on ice, centrifuged at 18,000 g for 30 min at 4°C and the soluble fraction was collected. For buffer D, bacterial pellet was harvested from 100 ml culture with OD of 1 and admixed with 2 ml cold lysis buffer I (Table 2) followed by harsh vortexing to solubilize all cell clumps. Lysozyme (Sigma-Aldrich, USA) was added to the lysate at a final concentration of 1 mg/ml and the suspension was incubated for 45 min at 4°C with gentle mixing. The suspension was quickly frozen by submerging in liquid nitrogen for 2 min, thawed immediately at 42°C, and blended by vortex vigorously to mix well. Four freeze-thaw-vortex cycles were performed. Sarkosyl (Sigma-Aldrich, USA) was added at a final concentration of 2% w/v, and the mixture was incubated at room temperature for 2 hr with gentle mixing. The cell lysate was centrifuged at 13000 g for 25 min at 4°C and supernatant was collected.
Large scale expression and purification of recombinant plac1: Based on the results, small scale expression of recombinant plac1 proteins was obtained and large scale protein expression experiment was carried out in the BL21 (DE3) strain. Pre-culture was performed in 15 ml TB medium at 37°C overnight. The culture was then diluted with 300 ml TB supplemented with 100 µg/ml ampicillin. Once OD600 reached 0.5-1, cold shock was initiated and expression was induced with 0.25 mM IPTG. Cells were further cultured for 24 hr at 15°C. The induced cells were harvested by centrifugation at 5000 g for 15 min at 4°C. Bacterial lysates were prepared with two lysis methods mentioned above and supernatants were collected and applied to Ni-NTA resin (Thermo Fisher Scientific, USA) on ÄKTA pure chromatography system (GE Healthcare, Germany). In brief, the resin was first equilibrated by equilibration/wash buffer (Na2HPO4:NaH2PO4 20 mM, NaCl 500 mM, Imidazole 30 mM, pH=7.4), the bacterial lysate was applied to the resin, washing the column was done with wash buffer and then elution was performed with elution buffer (Na2HPO4:NaH2PO4 20 mM, NaCl 500 mM, Imidazole 500 mM, pH=7.4). Finally, the eluted proteins were passed through the G-25 column for desalting and then lyophilized. The purity and molecular weight of the fractions containing purified main and control plac1 proteins was determined by SDS-PAGE gel electrophoresis under reducing condition. The expression of main and control plac1 and their purity were analyzed on a 12% SDS-PAGE gel stained with Coomassie brilliant blue G-250 (BioRad, USA). The protein concentration was determined by the BCA assay kit according to the manufacturer’s instruction.
Characterization of the recombinant proteins : To confirm the identity of main and control plac1 recombinant proteins, Western blotting and ELISA were performed. Since anti-plac1 antibody against mouse plac1 was not available, anti-human PLAC1 was used with confirmed reactivity with mouse orthologous genes 16. For Western blot analysis, 1 µg/well of each purified recombinant PLAC1 proteins was loaded on a 12% SDS-PAGE gel under reducing condition. Proteins were then transferred to nitrocellulose membrane (GE Healthcare, Germany) at 110 V for 75 min. Non-specific binding sites were blocked with 5% skim milk for 2 hr at room temperature with shaking. Membrane was washed 3 times with PBS containing 0.05% Tween 20 and blotted with 10 µg/ml rabbit anti-human PLAC1 for 1.5 hr at room temperature on shaker platform. After washing as mentioned above, membrane was incubated with 1:4000 dilution of HRP-conjugated sheep anti-rabbit Ig (Sina Biotech, Iran) for 1 hr. Bands were visualized using ECL system (GE Healthcare, Germany).
Reactivity of the purified proteins was also tested by ELISA. Briefly, the plate was coated with recombinant main and control plac1 proteins at final concentration of 5 µg/ml in PBS for 3 hr at 4ºC. Recombinant human PLAC1 was coated in control wells. Wells were then washed and blocked using PBS containing 3% Fetal Bovine Serum (FBS) at 37ºC for 2 hr. Serial dilutions of anti-recombinant human PLAC1 antibody from 10 µg/ml to 0.5 ng/ml were added and incubation was continued at 37°C for 1.5 hr. HRP-conjugated sheep anti-rabbit Ig (Sina Biotech, Iran) was then added to the wells at 1:2000 dilution and the reaction was revealed with 3,3′,5,5′-Tetramethylbenzidine (TMB) substrate. Optical density was measured by Multiskan™ FC Microplate (ELISA) Reader (Organon Teknika, Belgium) at 450 nm and 620 nm (As a reference wavelength).
Evaluation of antibody response against recombinant plac1 proteins: In order to evaluate the functionality of the produced recombinant plac1 proteins, they were injected subcutaneously into mice four times with 2-week intervals and sera were collected one week after each injection. For the first injection, 20 µg of antigen plus complete Freund’s adjuvant and for the next immunizations, 10 µg of antigen plus incomplete Freund’s adjuvant were used. Mice receiving adjuvant only served as the control group. The antibody titer in the immunized mice was assessed by ELISA. All steps were done as described above except that mice sera at serial dilutions of 1:200-1:25600 were used as a source of primary antibody and HRP-conjugated sheep anti-mouse Ig (Sina Biotech, Iran) at 1:2000 dilution was used as the detection antibody. This study was approved by the ethics committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.SPH.REC. 1399.078).
Statistical analysis: Comparison of anti-plac1 antibody titers between different groups at each serum dilution was performed using Mann-Whitney U test. Graphpad prism version 6.07 was employed for statistical analysis. The p-values less than 0.05 were considered statistically significant. ImageJ version 1.52v was used for calculation of relative band density of the generated recombinant proteins in bacterial cell lysate.
Results :
Construction of recombinant plac1 plasmid: Recombinant main plac1 construct (P2P30-PADRE-mouse plac1) with a length of 717 bp in pBSK (+) vector was transformed into the competent TOP10 E. coli. In selected colonies, amplified recombinant main plac1 yielded amplicon size of 900 bp using M13F and R primers. Main plac1 plasmid was then purified, subcloned in pColdi expression vector and transformed in the same E. coli strain. The amplified recombinant main plac1 PCR product yielded amplicon size of 850 bp using pColdi-specific universal primers. For preparation of control plac1 plasmid, recombinant main plac1 miniprep was digested with HindIII restriction enzyme and self-ligation was performed. Using pColdi-specific universal primers, amplicon size of 700 bp was confirmed using agarose gel electrophoresis. In both cases, the complete homology with the reference genome sequence was confirmed by sequencing before recombinant protein expression (data not presented).
Recombinant plac1 expression and purification: The minipreps encoding main and control plac1 were transformed into four E. coli strains and protein expression was induced. Different cultivation temperatures, cultivation time, IPTG concentration, and culture medium were studied and expression of the proteins in different conditions was evaluated by the SDS-PAGE. As expected, main and control plac1 showed bands of about 25 and 20 KD, respectively. The optimal expression level of main and control plac1 was obtained in BL21 (DE3) and TB culture medium in the presence of 0.25 mM IPTG after 24 hr of induction at 15°C (Figures 2 and 3). Also, our results showed that main and control plac1 were expressed as Inclusion Bodies (IBs) in BL21 (DE3) (Figure 4). Different buffers were tested to find the best condition for solubilization of inclusion bodies and the highest yield in purification of recombinant proteins. The results indicated that the highest level of soluble proteins was obtained when using IIA lysis buffer containing urea 8 M; however, proteins failed to attach to Ni-NTA resin in the presence of urea even when lower concentrations (2 M) of urea was used for protein solubilization. Similarly, proteins solubilized with 4-10% sarkosyl could not be purified over Ni-NTA resin. Finally, buffer containing 2% sarkosyl (Buffer D) was selected which resulted in the highest yield in protein purification. The purified proteins had high purity, as detected by SDS-PAGE (Figure 4). After purification, the eluted proteins were desalted using G-25 column with ÄKTA pure chromatography system (GE Healthcare, Germany) and desalted proteins were lyophilized. After purification, no obvious difference in molecular weight of main and control plac1 was observed and both recombinant proteins showed a molecular weight of about 25 KD. Based on BCA assay, approximately 600 and 800 µg of purified recombinant main and control plac1 proteins were obtained, respectively from 1 L of medium after desalting.
Characterization of the recombinant proteins: To confirm the identity of the purified proteins, Western blotting and ELISA assays were performed using anti-human recombinant PLAC1 polyclonal antibody. Our results showed specific reactivity of anti-human recombinant PLAC1 polyclonal antibody with both main and control plac1 recombinant proteins in ELISA (Figure 5A) and Western blot analysis (Figure 5B). In western blotting, specific bands of about 25 KD were observed. As expected, anti-human recombinant PLAC1 polyclonal antibody showed strong reactivity with purified human recombinant PLAC1 protein in ELISA yielding optical density of about 3 with as low as 30 ng/ml of coated protein. However, it showed weaker reactivity with main and control plac1 recombinant proteins.
Evaluation of antibody response against recombinant plac1 proteins: In order to evaluate whether or not the produced main and control plac1 recombinant proteins are able to elicit specific antibody response, mice were immunized with the purified proteins and the reactivity of the hyperimmune sera with the immunizing antigens was tested. The results showed that high antibody response was elicited even after the first injection in mice receiving main plac1 (Figure 6A). In control plac1 group, immunized mice produced lower ratio of specific antibody to immunizing antigen compared to the main plac1 group (p<0.0001). Sera from main plac1 group highly reacted with main plac1 (p<0.0001) at different dilutions (Figure 6B). Considering that main plac1 contained immunostimulatory peptides and in order to test specific antibody response against plac1, cross reactivity of the main plac1 sera was tested against control plac1. Our results showed that sera from main plac1 group exhibited higher reactivity against plac1 compared to control plac1 sera at all dilutions tested (p<0.05) (Figure 6C).
Discussion :
In this study, the optimized protocol was reported for production and purification of mouse recombinant plac1 protein. PLAC1 is among oncoplacental proteins with profound effects in cancer development and progression 3,5,6. To this end, PLAC1 has been regarded as one of the potential targets for cancer immunotherapy. Nonetheless, limited data are available on the efficient production of this antigen. The production of recombinant human PLAC1 has been already optimized 12; however, no study has been conducted so far on the production and purification of mouse plac1. In this study, an optimized protocol was reported regarding prokaryotic mouse plac1 production and purification. To induce higher levels of antibody responses, a recombinant plac1 protein containing immunostimulatory peptides, P2P30 and PADRE, was produced and humoral immune responses against it were compared to that of recombinant plac1 protein lacking those peptides.
Our results showed that production condition comprising BL21 (DE3) as host, TB as cultivation medium, induction with 0.25 mM IPTG for 24 hr, and using sarkosyl for solubilization gave the highest yield and purity. Although urea 8 M was able to solubilize large amounts of inclusion bodies leading to high yield recombinant plac1 proteins, these proteins failed to bind to Ni-NTA resin even at lower urea concentrations probably due to changes that urea exerts in the conformation of recombinant proteins. Sarkosyl also led to the solubilization of large amounts of inclusion bodies at high concentrations (4-10%), but like urea, the proteins prepared in this method did not attach to the Ni-NTA resin. Sarkosyl at higher concentrations could change the structure and conformation of recombinant proteins as well 17. Although 2% sarkosyl was less effective in solubilizing inclusion bodies, it did not considerably interfere with recombinant plac1 binding to Ni-NTA resin.
As expected, main plac1 showed a relatively less electrophoretic mobility compared to control plac1 (25 vs. to 20 KD). However, after solubilization of urea or sarkosyl, this molecular weight difference was visually lost, which could probably be attributed to structural changes in control plac1 conformation following exposure to these potent detergents leading to a shift in electrophoretic mobility. In order to replace the buffer and remove imidazole from the purified proteins, desalting with chromatography system was applied instead of dialysis, which greatly reduced the amount of protein loss.
It was also shown that both recombinant plac1 proteins specifically reacted with anti-recombinant human PLAC1 antibody in Western blotting and ELISA confirming the identity of the generated proteins. As expected, anti-recombinant human PLAC1 antibody showed considerably higher reactivity with human PLAC1 compared to our generated mouse plac1 proteins. This is because human and mouse PLAC1 proteins show only 60% identity at the protein level. To demonstrate the capacity of produced recombinant plac1 proteins to induce humoral responses, these proteins were injected into mice and the levels of specific anti-plac1 antibodies were measured. Several studies have shown that PLAC1 could induce humoral responses in patients with different types of cancers. Fourteen out of 226 plasma samples (6%) from NSCLC patients contained detectable levels of anti-PLAC1 antibodies 18. Six out of the 20 (33%) patients with PLAC1 mRNA positive colorectal cancers produced anti-PLAC1 antibodies 7. Notably, pregnancy itself could also be considered as a trigger for induction of anti-PLAC1 antibodies. Four out of 78 (5%) healthy adults with previous history of pregnancy showed seropositivity with recombinant PLAC1 protein (Amino acids 125–212) 18. In line with these studies, plac1 protein was shown to induce humoral responses in vivo. As expected, however, the placement of P2P30 and PADRE peptides next to the plac1 resulted in an increased immunogenicity and enhanced plac1-specific humoral responses most probably through polyclonal activation of helper T cells (Th) 13,14. Presenting P2P30 and PADRE epitopes by MHCII molecules of B lymphocytes as antigen presenting cells to Th cells leads to the activation of these cells, resulting in production of cytokines including interferon-γ and efficient binding of CD40L to CD40 at the surface of PLAC1-specific B lymphocytes, which is the second signal for the proliferation and differentiation of PLAC1-specific B lymphocytes , potentially leading to generation of antibodies with higher affinity 19. Application of tetanus toxin c-fragment (TTfrC), especially its first domain (pDome), which contains P2P30 activated all components of the specific immune system including CD4+, CD8+ T cells, and B cells in patients with follicular lymphoma and multiple myeloma 20. Inserting P2P30 and PADRE into human TNFα gene structure induced production of antibodies against TNFα, which efficiently neutralized biological activity of TNFα in vitro 21. Similarly, placement of PADRE and 4 epitopes of tetanus toxin fragment c (TTfrc) along with WT1 and human papillomavirus E7 antigens induced high IgG titers, reduced the number of pulmonary metastatic nodules, and increased survival compared with controls in a mouse model of TC-1 tumor 22.
In this study, four bacterial strains, one vector, and a limited set of solubilization and purification procedures were tested. Different bacterial strains, expression vectors, and purification processes are needed to further improve yield and purity of the plac1 protein.
Conclusion :
In this study, an optimized protocol was reported for the production and purification of functional mouse recombinant plac1 protein and it was shown that insertion of immunostimulatory peptides in gene construct could efficiently enhance humoral immune responses against this protein. This could potentially augment cellular immune responses against plac1 leading to more effective anti-cancer responses. Generated proteins have broad applications for studying PLAC1 immunobiology and immunotherapy of tumors with PLAC1 expression.
Acknowledgement :
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [982730] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran and in part by Tehran University of Medical Sciences (TUMS) (Grant No: 98-02-27-42488), Tehran, Iran.
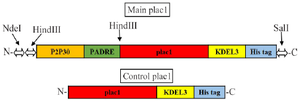
Figure 1. Schematic representation of main and control plac1 constructs. Restriction enzyme recognition sites are shown by arrows.
|
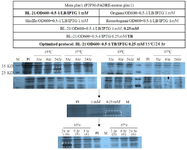
Figure 2. Optimization of main plac1 expression in prokaryotic system. In the first step, the bacterial strain was selected under the same conditions. In the next step, the IPTG concentration was set up. Finally, the bacterial culture medium and cultivation time and temperature were selected. Optimized conditions are shown in bold letters. SDS-PAGE analysis was performed using 12% polyacrylamide gel and Coomassie Blue R-250 stain. Specific bands are shown by dashed rectangles. The relative density (percentage) of the desired protein in the optimized condition is shown in parenthesis.
PI: Pre-induction, M: Protein marker.
|

Figure 3. Optimization of control plac1 expression in prokaryotic system. The optimization steps were the same as with main PLAC1 (Figure 2). Optimized induction time and temperature were selected based on the optimal parameters of main PLAC1 expression. SDS-PAGE analysis was performed using 12% polyacrylamide gel and Coomassie Blue R-250 stain. Specific bands are shown by dashed rectangles. The relative density (percentage) of the desired protein in the optimized condition is shown in parenthesis.
PI: Pre-induction, M: Protein marker.
|

Figure 4. Purification of main and control plac1 with different lysis buffers. Different lysis buffers (a-g) were used for solubilization and purification of recombinant main (A) and control plac1 (B). Composition of each buffer (a-g) is presented in table 2.
PI: Pre-induction, M: Protein marker, F: Fraction
|
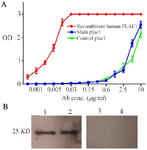
Figure 5. Characterization of recombinant main and control plac1 proteins. Reactivity of main and control plac1 proteins was tested by ELISA and Western blotting. A) Wells of ELISA plate were coated with main or control plac1. In control wells, recombinant human PLAC1 was coated. Reactivity of the coated proteins with polyclonal anti-human PLAC1 antibody was tested by ELISA in triplicate. B) Reactivity of main (Lane 1) and control plac1 (Lane 2) with polyclonal antibody against recombinant human PLAC1 was confirmed by Western blotting. In negative control lanes (Lanes 3 and 4), primary antibody was omitted. Error bars show standard error of mean.
|
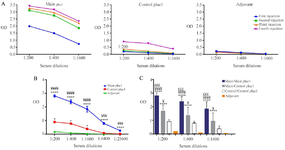
Figure 6. Evaluation of antibody response against recombinant main and control plac1 proteins. Mice were immunized with either main or control plac1. Mice receiving adjuvant only served as the control group. A) Specific antibody titers were determined one week after each immunization. B) Reactivity of the titrating dilutions of hyperimmune sera from different groups (each containing 6 mice) after the fourth immunization was tested in triplicate with cognate antigen as a coating layer. Sera of adjuvant group were only tested with control plac1. C) Cross-reactivity of the main plac1-immunized sera collected after the fourth immunization with control plac1 was tested in triplicate. Each group contained 6 mice. Error bars show standard error of mean.
*: Statistical comparison with adjuvant group, ¥: Statistical comparison with control plac1 group, £: Statistical comparison with main/control plac1 group.*, ¥: p<0.05, £££, ¥¥¥: p<0.001, ****, ¥¥¥¥: p<0.0001
|

Table 1. Primers used for amplification of main and control plac1
|
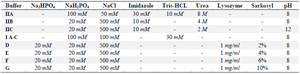
Table 2. Composition of the lysis buffers used in solubilization of inclusion bodies
|
|