Cytotoxic Activities of Silver Nanoparticles and Silver Ions in Parent and Tamoxifen-Resistant T47D Human Breast Cancer Cells and Their Combination Effects with Tamoxifen against Resistant Cells
-
Ostad, Seyed Naser
-
Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Dehnad, Shahrzad
-
Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Pharmaceutical Research Division, Azad Islamic University , Tehran, Iran
-
Esmail Nazari, Zeinab
-
Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Tavajohi Fini, Shohreh
-
Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Mokhtari, Narges
-
Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
Shakibaie, Mojtaba
-
Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
-
 Shahverdi, Ahmad Reza
Ph.D., Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran, P.O.Box: 6451-14155, Tel: +98 21 66482706 Fax: +98 21 66461178 E-mail: shahverd@tums.ac.ir
Shahverdi, Ahmad Reza
Ph.D., Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran, P.O.Box: 6451-14155, Tel: +98 21 66482706 Fax: +98 21 66461178 E-mail: shahverd@tums.ac.ir
-
Department of Pharmaceutical Biotechnology, Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences , Tehran, Iran
Abstract: Studies on biomedical applications of nanoparticles are growing with a rapid pace. In medicine, nanoparticles may be the solution for multi-drug-resistance which is still a major drawback in chemotherapy of cancer. In the present study, we investigated the potential cytotoxic effect of silver nanoparticles (Ag NPs) and silver ions (Ag+) in both parent and tamoxifen-resistant T47D cells in presence and absence of tamoxifen. Ag NPs were synthesized (< 28 nm) and MTT assay was carried out. The associated IC50 values were found to be: 6.31 ?g/ml for Ag NPs/parent cells, 37.06 ?g/ml for Ag NPs/tamoxifen-resistant cells, 33.06 ?g/ml for Ag+/parent cells and 10.10 ?g/ml for Ag+/resistant cells. As a separate experiment, the effect of subinhibitory concentrations of Ag NPs and Ag+ on the proliferation of tamoxifen resistant cells was evaluated at non-toxic concentrations of tamoxifen. Our results suggested that in non-cytotoxic concentrations of silver nanomaterials and tamoxifen, the combinations of Ag+-tamoxifen and Ag NPs-tamoxifen are still cytotoxic. This finding may be of great potential benefit in chemotherapy of breast cancer; since much lower doses of tamoxifen may be needed to produce the same cytotoxic effect and side effects will be reduced.
Introduction :
The past few years witnessed an increasing attention towards rapid development in every aspect of what is called "nanotechnology". Nanotechnology is briefly termed as "The ability to fabricate, characterize, and manipulate artificial structures, whose features are controlled at the nanometer level" (1). This may involve design, synthesis and preparation of nanoparticles to create products with novel properties (2 - 4). In recent years, there has been much interest in the application of nanomaterials for biological purposes. These materials include nanoparticles (5 - 7), nanotubes (8), nanowires (9), and quantum dots (10). Nevertheless, the biomedical applications of nanomaterials as cytotoxic or antimicrobial agents, biosensors and, drug carriers are growing with a rapid pace (11 - 13).
Despite many efforts, multi drug resistance is still considered as a major drawback in chemotherapy of cancer which has been the subject of exhaustive experiments in this area (14). The cellular mechanisms underlying this phenomenon are well-discussed before (15). It is also well-known that one of the main mechanisms underlying drug resistance is based on the expression of MDR-1 gene which leads to the formation of membrane-bound ABC transporter proteins, such as P-glycoproteins (16, 17). Alternative mechanisms include detoxification processes by cytochrome-P450 metabolism enzymes and glutathione, cellular repair mechanisms which repair drug-induced DNA damages, and alteration of apoptotic signaling pathways, so that cells could resist drug-induced apoptosis.
Tamoxifen is a frequently-prescribed medication, widely used in all stages of breast cancer (18). However, tamoxifen-resistance is still a major problem which limits its application in chemotherapy of breast cancer (19, 20). In order to reduce tamoxifen-resistance, prodigious efforts have been made on combination therapies of tamoxifen and different compounds, such as gap junctional activator (21). The combination therapy of drugs and nanoparticles is promising since it may improve the penetration of drugs into tumor cells, enhances their ability of tumor targeting, and reduces their side effects (22). Recently, the cytotoxic effect of tamoxifen-loaded polymeric nanoparticles was investigated by Amiji and coworkers (23).
The biological applications of silver nanoparticles (Ag NPs) have been widely studied; antimicrobial properties in particular (24 - 27). Ag NPs are known to be cytotoxic to both normal and cancer cells in mammals (28) and the modes of interactions of Ag NPs have been investigated in different prokaryotic and eukaryotic systems (29 - 31). The cytotoxic effects of silver ions (Ag+) have been reported in different cell lines as well (32, 33). However, no comparative experiment has been yet carried out on cytotoxic effects of the combination of silver nanomaterials and tamoxifen on cancer cell lines. In addition, along with promising cytotoxic effects of silver nanomaterials, much concern has lately been raised about the safety issue of these compounds due to undesirable toxic effects of Ag NPs on both human health and environment (34, 35).
This study represents a comparative in vitro evaluation of the effect of Ag NPs and Ag+ on the viability of two cell subtypes; human T47D breast cancer cell line and tamoxifen resistant T47D cell line. Also, the cytotoxic effect of subinhibitory concentrations of Ag NPs and Ag+ on tamoxifen-resistant cells both in presence and absence of tamoxifen was investigated.
Materials and Methods :
Materials
RPMI-1640 and fetal bovine serum were purchased from Gibco (United States); 3-(4,5-dimethylthiazol-2-yl) -2,5-diphenyl tetrazolium bromide (MTT), tamoxifen, penicillin, and streptomycin from Sigma (United Kingdom); and dextrose, silver nitrate (AgNO3), sodium hydroxide (NaOH), hydrogen chloride (HCl), and isopropanol from Merck (Germany).
Synthesis of Ag NPs
The chemical reduction of an aqueous solution of AgNO3 is one of the most widely-used methods for synthesis of Ag colloids (36). An aqueous solution containing 0.5 mM Ag+ was prepared by adding 22.5 mg of dextrose to 100 ml of AgNO3 solution (0.5 mM). This solution was then alkalized using 20 µl of 0.1 N NaOH and treated in a microwave oven for 60 sec to induce the reduction of metal ions. The reduction of the Ag+ by dextrose in the solution was monitored by sampling the aqueous component (2 ml) and then measuring the ultraviolet visible (UV-Vis) spectra of the solutions on a UV-Vis double-beam automatic-scanning spectrophotometer (Labomed, Inc., Spectro UV-Vis Double PC, Model UVD-2950), operated at 2 nm resolution. Furthermore, Ag NPs were characterized by Transmission Electron Microscopy (TEM) (Phillips CM200 field emission gun) and Energy-Dispersive Spectroscopy (EDS). For this propose, an aqueous suspension containing the Ag NPs was dispersed ultrasonically, and a drop of the suspension was placed on carbon-coated copper TEM grids and dried under an IR lamp. Micrographs were obtained using a TEM operated at an accelerating voltage of 80 kV.
Cell line and culture conditions
The T47D human breast cancer cell line (ATCC HTB-133, United States) was purchased from the National Cell Bank of Iran (Pasteur Institute of Iran, Tehran, Iran). The subline of the tamoxifen-resistant T47D human breast cancer cell line was a gift from the Molecular Research Laboratory, Department of Pharmacology and Toxicology, Faculty of Pharmacy, Tehran University of Medical Sciences (Tehran, Iran) (37)¬. Parent cancer cell line was maintained in RPMI-1640 culture medium supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 µg/ml streptomycin in a 5% carbon dioxide (CO2) cell incubator at 37 °C. The tamoxifen-resistant cancer cell line was cultured in the described medium supplemented with 1 µM of tamoxifen and maintained under the same conditions of parent cancer cells.
Cytotoxicity assay
MTT-based assay was performed according to a previously-described method (38) by preliminarily seeding of 45,000 cancer cells in a 100 -µl growth medium in the presence of increasing concentrations of Ag NPs and Ag+ (15, 20, 30, 40, 45, and 50 µg/ml) in 96-well plates, individually and subsequent incubation of cells at 37 °C in 5% CO2 for 48 hr. The cells were thereafter treated with 25 µl of MTT (5 mg/ml) and incubated at 37 °C for 4hr. Nontreated cells were used as control. After dissolving formazan crystals in 0.04 N HCl in isopropanol, 96-well plates were read in a microplate reader (Dynatech Laboratories, Inc, United States) at 570 nm. Each experiment was repeated three times and the MTT assay was performed in triplicate for each experiment. Cytotoxicity was calculated as the percentage of viable cells at different concentrations of samples relative to the control (untreated) cells. Also, the half maximal Inhibitory Concentration (IC50) was calculated as the concentration required inhibiting the growth of tumor cells in culture by 50% compared to the untreated cells.
As a separate experiment, the combination effect of Ag NPs and Ag+ was studied both in presence and absence of tamoxifen, in tamoxifen-resistant T47D cells. Subinhibitory concentrations of Ag NPs (2.5 µg/ml) and Ag+ (1.5 µg/ml) were determined by a preliminary MTT assay on tested cell lines. The effects of these samples were investigated at the concentrations mentioned above on the proliferation of tamoxifen resistant T47D cells grown in both tamoxifen-containing (1 µM) and tamoxifen free culture media
Result :
Synthesis of silver nanoparticles and their characterization
In this study, the Ag NPs were synthesized using the chemical reduction method described earlier (36). The synthesized Ag NPs were subsequently characterized by UV-Vis spectroscopy (Figure 1). It is worth-mentioning that the technique outlined above, proved to be very useful in analysis of nanoparticles. As illustrated in figure 1, the strong absorption band with a maxima located at 420 nm was caused by the formation of Ag NPs produced by the dextrose. This peak is attributed to the surface plasmon phenomenon which is well identified in various metal nanoparticles with sizes ranging from 2 nm to 100 nm (39, 40). The inset to figure 1 is a photograph of as-prepared Ag colloids.
Figure 2A, shows representative TEM image recorded from the drop coated film of the Ag NPs synthesized by treating the Ag NPs solution with the dextrose in a microwave oven. At least 200 randomly selected nanoparticles from TEM image were used to illustrate a particle size distribution histogram. The particle-size histogram of Ag NPs produced by dextrose (Figure 2, on the right) indicates that Ag NPs vary in size from 1 nm to 28 nm. Most Ag NPs (approximately 90%) obtained by this method varied in their size from about 1 nm to almost 12 nm. EDS analysis of the Ag NPs confirmed the presence of an elemental Ag signal in the sample (Figure 3). Ag nanocrystallites displayed an optical absorption band with a peak at 3 keV, which is typical for the absorption of metallic Ag nanocrystallites (copper and carbon peaks existed due to the grid used for TEM imaging) (30).
Cytotoxicity assay of silver nanoparticles and its combination with tamoxifen
The cytotoxic effect of Ag in both forms of Ag+ and Ag NPs was evaluated in vitro in both parent and tamoxifen-resistant T47D human breast cancer cell lines at different concentrations of 15, 20, 30, 40, 45, and 50 µg/ml. As indicated in Figure 4, both parent and tamoxifen-resistant cells shows different patterns of dose-dependent responses in presence of metallic and ionic forms of silver nanomaterials. In detail, at the low concentration of 15 µg/ml Ag NP, a significant cytotoxic effect was observed which reduced the percentage of viable cells from 100% to 25%, followed by a moderate decrease from 25% at the concentration of 15 µg/ml Ag NPs, to less than 10% at 15 µg/ml concentration (Figure 4A). However, in the tamoxifen-resistant T47D cells, no more than 10% decrease in the proportion of viable cells was observed at concentrations below 30 µg/ml of Ag NPs, at which, the percentage of viable cells slowly decreased from 50% to 40% (Figure 4B).
Figures 4C and 4D resemble the behaviors of parent and tamoxifen-resistant cells in presence of ionic – instead of metallic – form of silver. In the presence of Ag+, the viability of parent cells decreased in an almost linear manner, from 90% at 15 µg/ml concentration to about 20% at the maximal 50 µg/ml concentration (Figure 4C), whilst an essentially different pattern was observed for tamoxifen-resistant T47D cells. The proportion of viable tamoxifen-resistant cells fell down to 40 percent at the meager concentration of 15 µg/ml and mildly decreased from 40 to about 17% at 50 µg/ml concentration (Figure 4D).
The IC50 values associated with Ag NPs and Ag+ in both cell lines are shown in table 1. The concentrations needed to produce 50% cell death were 37.06 µg/ml for the Ag NPs and 10.1 µg/ml for the Ag+ on the tamoxifen-resistant T47D cell lines, whereas 6.31 -µg/ml and 33.06 -µg/ml concetrations of Ag NPs and Ag+ produced the same effect on parent T47D cells (Table 1).
In a separate experiment, the effect of subinhibitory concentrations of Ag NPs (2.5 µg/ml) and Ag+ (1.5 µg/ml) on the proliferation of tamoxifen-resistant T47D human breast cancer cells was investigated both in presence and absence of sub inhibitory concentration of tamoxifen (1 µM). Figure 5 shows the effect of Ag NPs (Figure 5A) and Ag+
Discussion :
The antiproliferative effect of Ag NPs and Ag+ is evident (41 - 44). Furthermore, the interaction of Ag NPs and Ag+ with certain proteins has been the subject of extensive studies in recent years. In this study, antiproliferative effect of silver – in both ionic (Ag+) and metallic (Ag NPs) forms – on parent and tamoxifen-resistant cells was investigated. Cells were observed to exhibit different responses after treatment with silver nanomaterials. the associate IC50 values – as indicated in table 1, were 6.31 µg/ml for parent cells in presence of AgNPs, 37.06 µg/ml for tamoxifen-resistant cells in presence of Ag NPs, 33.06 µg/ml for parent cells in presence of Ag+, and 10 µg/ml for tamoxifen-resistant cells in presence of Ag+.
The results primarily imply that silver – either in ionic or metallic forms – have cytotoxic effects on both parent T47D and tamoxifen-resistant cells. Moreover, it appears from table 1 that parent cells were more susceptible to Ag NPs, while in reverse, tamoxifen-resistant cells were more susceptible to Ag+ rather than Ag NPs. In parent T47D cells, the reason for enhanced cytotoxicity of Ag NPs (6.31 µg/ml) compared with Ag+ (33.06 µg/ml) may be due to the relative stability of Ag NPs which facilitates their subsequent penetration and survival in tumor cells. Therefore, Since Ag+ possess low stability and more reactivity, it is likely that silver ions undergo further deionization and other unwanted reactions before reaching tumor cells.
On the other hand, the condition is not the same in tamoxifen-resistant cells due to numerous substantial differences between parent and resistant cells. As mentioned earlier, several mechanisms are involved in multi drug resistance of cells, among which, the pumping activity of ABC transporters such as P-glycoproteins – located in the cell membrane- is of particular importance. Therefore, assuming membrane glycoproteins as a major obstacle for the internalization of silver-based nanomaterials into resistant cells, it is probable that Ag+ may has easily penetrated into tamoxifen-resistant cells, while Ag NPs have been pumped out of the cells by P-glycoproteins. In other words, although nanomaterials have been shown to easily pass through cell membranes (45), the active reflux of Ag NPs from cells may hinder its maintenance in cells, thereby limiting its cytotoxic effect from IC50=6.31 µg/ml in parent cells to IC50= 37.6 µg/ml in tamoxifen-resistant cells.
However, after internalization of Ag NPs and Ag+ into cells (which resembles the situation in parent cells), the cytotoxic effects of Ag NPs is more than that of Ag+, probably due to the fact that Ag NPs may interfere with the proper functioning of cellular proteins and induce subsequent changes in cellular chemistry; as proposed previously by Rogers et al. (42). This hypothesis is also in good agreement with experiments of Zolghadri and coworkers (46) where they demonstrated that Ag NPs provide a relatively high hydrophobicity inside bovine hemoglobin which causes a transition from alpha helixes to beta sheets and leads to partial unfolding and aggregation of the protein (46). Other experiments suggest that Ag NPs are likely to interact with thiol-rich enzymes (47). Therefore, it sounds possible that once penetrated into cells, silver nanoparticles may attack functional proteins of cells which results in partial unfolding and aggregation of proteins as it is the case in the bovine hemoglobin.
Figure 5 is a graphic illustration of the cytotoxic effects of subinhibitory concentrations of Ag NPs (A) and Ag+ (B) in presence and absence of subinhibitory concentrations of tamoxifen in tamoxifen-resistance cells. It should be pointed out that the tamoxifen concentration of 1 µM was chosen to guarantee that the effect produced was due to the combination and not to the effect of the tamoxifen itself. So the effect observed in this condition could be due to the inorganic materials–tamoxifen combination. The bar graph clea
Acknowledgement :
This work was financially supported by a grant (No: 85-04-33-4778) from the Deputy of Research, Tehran University of Medical Sciences, Tehran, Iran. Also the support of the Iranian Nanotechnology Initiative is also gratefully acknowledged.
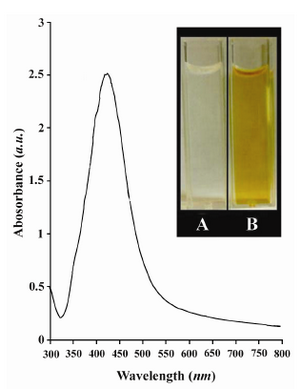
Figure 1. UV-Vis spectrum of as-prepared Ag NPs synthesized by chemical reduction of Ag+ by dextrose. The inset is A) a photograph of the reaction mixture before reduction and B) after reduction
|
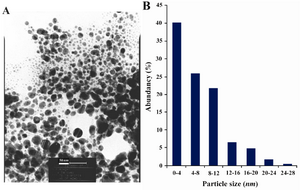
Figure 2. A) TEM recorded from a small region of a drop-coated film of AgNO3 solution treated with dextrose for 60 sec in a microwave oven (the scale bars correspond to 50 nm). B) The related particle-size distribution histogram was obtained after counting 400 individual particles
|
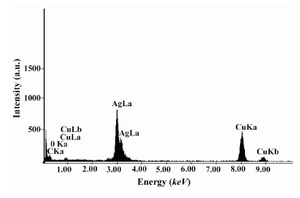
Figure 3. EDS spectra of prepared Ag NPs. Ag x-ray emission peaks are labeled. The strong signals from the atoms in the nanoparticles shown in the spectrum confirm the reduction of Ag+ to Ag NPs
|
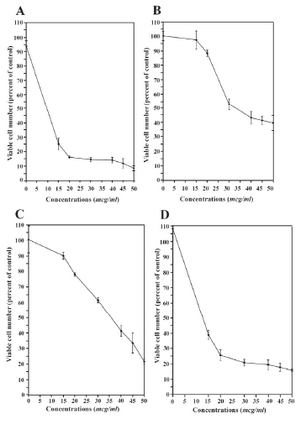
Figure 4. The effects of Ag+ and Ag NPs on the cells: A) Ag NPs on parent T47D cells, B) Ag NPs on the 1�10-6 M tamoxifen-resistant T47D cells, C) Ag+ on the parent T47D cells and D) Ag+ on the 1�10-6 M tamoxifen resistant T47D cells (n = 6)
|
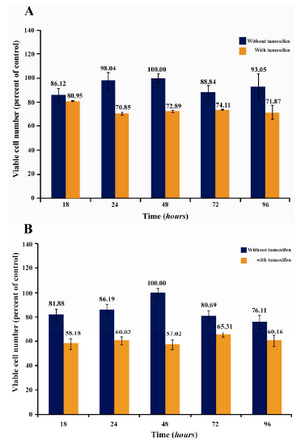
Figure 5. The combined effect of subinhibitory concentrations of Ag NPs (A) and Ag+ (B) with tamoxifen (1 �M) on tamoxifen-resistant T47D human breast cancer cells
|
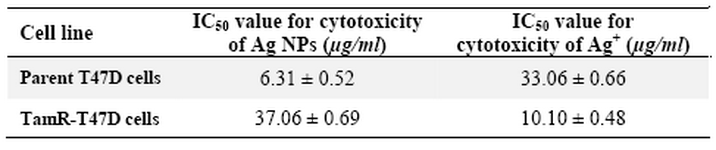
Table 1. The inhibitory potential of the Ag NPs and Ag+ on the viability of parent T47D human breast cancer cells and its tamoxifen-resistant cell subline (TamR) (sample size = 6, repetitions = 3, calculated p < 0.05)a
a IC50 was calculated using Sigmaplot software. IC 50 for parent T47D cells and tamoxifen resistant T47D cell sub line were obtained > 2.5 �M. Tamoxifen was used at concentration of 1 �M in all experiments
|
|