Fabrication of Calcium Sulfate Coated Selenium Nanoparticles and Corresponding In-Vitro Cytotoxicity Effects Against 4T1 Breast Cancer Cell Line
-
Faghfuri , Elnaz
-
Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Ajideh, Ramak
-
Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Shahverdi , Faranak
-
Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Hosseini, Mina
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Mavandadnejad, Faranak
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Yazdi, Mohammad Hossein
-
Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
 Shahverdi, Ahmad Reza
Department of Pharmaceutical Biotechnology and Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran, Tel/Fax: +98 21 66482706; E-mail: shahverd@sina.tums.ac.ir
Shahverdi, Ahmad Reza
Department of Pharmaceutical Biotechnology and Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran, Tel/Fax: +98 21 66482706; E-mail: shahverd@sina.tums.ac.ir
-
Biotechnology Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
-
Recombinant Vaccine Research Center, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: The inhibitory effect of selenium nanoparticles (SeNPs) on cancer cells has been reported in many studies. In this study, the purpose was to compare the in vitro effects of SeNPs and calcium sulfate coated selenium nanoparticles (CaSO4@ SeNPs) on breast cancer cells.
Methods: CaSO4@SeNPs and SeNPs were chemically synthesized and characterized with Field Emission Scanning Electron Microscope (FESEM) and energy-dispersive X-ray spectroscopy (EDX). By applying MTT assay, the cytotoxicity effect of both nanomaterials on the 4T1 cancer cells was investigated.
Results: While LD50 of SeNPs on 4T1 cancer cells was 80 µg, the LD50 of CaSO4@SeNPs was reported to be only 15 µg. The difference between the inhibition rates obtained for SeNPs and CaSO4@SeNPs was statistically significant (p=0.05). In addition, at higher concentrations (50 µg) of CaSO4@SeNPs, the cytotoxicity was 100% more than SeNPs alone.
Conclusion: According to the result of the present work, it can be concluded that decoration of SeNPs with calcium sulfate leads to an increase in potency by decreasing the effective dose. This effect can be attributed to activation of intrinsic apoptosis signaling and/or pH regulatory properties of CaSO4@SeNPs. However, further studies are still needed to determine the exact corresponding mechanisms of this synergistic effect.
Introduction :
Besides conventional treatments such as chemo-therapy and radiotherapy and the latest breakthroughs in molecular biology and immunotherapy in cancer treatment, breast cancer is the most common type of female cancer worldwide representing nearly a quarter (23%) of all cancers in women and a significant challenge to public health 1. Despite significant prog-resses in the management of preventive or therapeutic modalities to control this cancer type, the search for a curative treatment is still ongoing 2-4.
Selenium (Se), a vital micronutrient with proven benefits for human biology, is obtained from dietary sources. This trace element has many important biological effects, particularly chemo preventative and therapeutic properties 5,6. Small amounts of selenium are vital for specific biological functions in humans. As it is an integral part of glutathione peroxidase and thioredoxin reductase enzyme, it is capable of scavenging free radicals and regulating the function of the thyroid gland. Additionally, it has been found to be involved in fertility improvement and ensures proper functioning of the immune system 7-9. The use of elemental selenium, an insoluble metalloid compound produced at nano-scale, chemically or biologically is gaining a great deal of attention due to low therapeutic index of organic and inorganic selenium 10,11. Various studies have shown that Se supplementation reduces the incidence of prostate, lung, and colon cancers 11-13. Selenium Nanoparticles (SeNPs) are known as a metalloid form of Se species which have been reported as potential cancer therapeutic agents and drug carriers 14-17.
In our previous researches, the indirect effect of SeNPs on augmentation of immune responses against breast cancer tumor cells and enhancing the lifespan of tumor bearing animals was demonstrated 18. The mechanism of apoptosis induction has attracted researchers' attention for fighting against cancer 19-21. On the other hand, applying a non-vehicular system for delivering apoptogenic agents to cancer cells to induce apoptosis has been investigated 20. Moreover, the direct cytotoxicity of SeNPs with different surface modific-ation forms has been reported against different cell lines in recent years 22,23. For instance, in our recent study, FA@SeNPs, a nanocomposite consisting of SeNPs and folic acid, indicated a considerable potential to target cancer cells 22. On the other hand, calcium has been remarked for anti-cancer therapy because of its availability, low cost, safety, outstanding biocompati-bility, pH-sensitivity, and slow biodegradability 20,24,25. Calcium could have toxic effect on cancer cells through triggering apoptosis by increasing the pH of tumor microenvironment that can sensitize the drug resistant cancer cells 26.
There are always risks and adverse effects of administering chemicals, drugs, and medicine via nano carriers. In this study, the purpose was to reduce such risks regarding the properties of calcium and also its possible synergistic effect with SeNPs to eradicate cancer cells. Additionally, the cytotoxicity of CaSO4@ SeNPs on breast cancer cells was highlighted.
Materials and Methods :
Fabrication of SeNPs and CaSO4@SeNPs: One-step method with some modifications was used to synthesize calcium sulfate coated selenium nano-particles (CaSO4@SeNPs) according to the previous report 27. In the first step, by applying a well-known method, SeNPs were synthesized 22. For this purpose, 4.8 ml of ascorbic acid in aqueous solution (50 mM) was gradually added to the 100 ml selenium dioxide solution (50 mM) by continuous stirring (300 rpm). At that point, the mixture was centrifuged and washed three times with double-distilled water. For preparing CaSO4@SeNPs, plain SeNPs (160 mg) were re-suspended in 50 ml sodium sulfate solution (25 mM). In the next step, 20 ml calcium chloride (350 mM) was added drop by drop into the mixture with continuous stirring (300 rpm). Finally, the reaction mixture was centrifuged and washed three times with double-distilled water. Stock suspension of SeNPs and CaSO4@SeNPs was prepared (10 mg/ml) and used for additional cytotoxicity assays.
Characterization of prepared NPs: To observe the NPs’ surface features and determine the elemental composition, a Field Emission Scanning Electron Microscope (FESEM) equipped with Energy-Dispersive X-ray Spectroscopy (EDS) was used. For FESEM observation, NPs were mounted on specimen stubs and coated with gold. Samples were analyzed with MIRA 3 FESEM (TESCAN MIRA3, USA) operated at 15 kV, and EDS was recorded by focusing on a cluster of NPs.
Cell culture: The 4T1 breast cancer cell line (Product code: ATCC CRL-2539) was purchased from the National Cell Bank of Iran (NCBI), Pasteur Institute of Iran, Tehran, Iran. The RPMI 1640 medium with 10% Fetal Bovine Serum (FBS), 100 u/ml penicillin, and 100 µg/ml of streptomycin was prepared. The 4T1 cancer cells were cultured in this medium in the condition of 5% CO2 at 37°C for 1 week. For the cytotoxicity assay, the serum starvation condition was applied by using fetal bovine serum-free RPMI 1640 medium containing the antibiotics mentioned above and the cells were re-incubated at 37°C for 18 hr (5% CO2).
Cell cytotoxicity assay: To evaluate cell cytotoxicity, thiazolyl blue tetrazolium bromide (MTT) assay was used and 2×104 starved cells were seeded in 96 well plates and incubated with 12.5, 25, 50, 100, and 200 µg of CaSO4@SeNPs and SeNPs for 24 hr. Thereupon, 15 μl MTT (0.5 mg/ml) was added to each well, and the plates were incubated for 2 hr. After that, the medium was replaced with 200 μl of Dimethyl Sulfoxide (DMSO), and the absorbance was recorded at 570 nm 28. Each experiment was repeated three times, and the MTT assay was performed in three replicates for each experiment. The percentage of viable cells was measured using the following equation, Viable cells (%)=[ODexp/ODcon]×100, where ODexp and ODcon are the optical densities of the treated and untreated cells, respectively. Finally, the cytotoxicity bar histogram was obtained by plotting the percentage of viable cells against the concentration of cytotoxic compounds.
Statistical analysis: The Holm-Sidak multiple comparison test was used for statistical analysis followed by one-way analysis of variance (ANOVA). Shapiro-Wilk test was used to examine normal distribution of data and Levene's test showed the homogeneity of the variances between the compared groups. The values are reported as mean± Standard Deviation (SD), and a p-value of less than 0.05 is considered statistically significant.
Results :
Preparation and characterization of SeNPs and CaSO4@ SeNPs:SeNPs were chemically synthesized and their shape and size were confirmed by FESEM, as shown in figure 1A. The FESEM image showed that the particles size range was below 100 nm, with an average size of about 60 nm. In addition, the FESEM image of the prepared CaSO4@SeNPs in figure 1B indicated that the generated nanocomposites were rod shaped (400×100 nm). The EDS spectra of the SeNPs and CaSO4@ SeNPs are demonstrated in figures 2 and 3, respectively. It reveals the presence of Se element peaks. These EDS peaks confirm the existence of elemental SeNPs in both types of prepared NPs. Additional peaks related to calcium, copper, carbon, oxygen, and nitrogen elements are attributes of the grid used for EESEM imaging or calcium sulfate substance.
Cytotoxicity effects: Cytotoxicity effects of both CaSO4@SeNPs and SeNPs were evaluated in vitro at different concen-trations (10, 25, 50, 100, and 200) on 4T1 breast cancer cells. The inhibition rate of CaSO4@SeNPs increased at higher concentrations which was shown by cytotoxicity analysis of different doses with the aid of statistical data analysis (p<0.05) (Figure 4). The considerable concentrations that led to 50% cell death (IC50) were reported to be approximately 80 µg in the cells treated with SeNPs. On the other hand, the CaSO4@SeNPs caused the same effect at the concentration of 15 µg. In this study, the possible combinatorial effect of CaSO4 with SeNPs to kill breast cancer cells was significant at the doses of 25 and 50 µg. In other words, although the doses of SeNPs should be 25 or 50 µg to show 40% inhibitory effect, equal doses of CaSO4@SeNPs show two-fold higher impact on killing cancer cells (Nearly 80% of IR). In 100 µg and 200 µg doses of SeNPs and CaSO4@SeNPs, the combinatorial effect decreased, which may be due to particle agglomeration at higher concentrations of SeNPs.
Discussion :
Previous researches demonstrated that different types of selenium have various benefits for human body 29. The biological impacts of Se mostly depend on the incorporation of this metalloid into seleno-proteins in the form of seleno-cysteine amino acid 30. However, many past and recent clinical trials are trying to use selenium and its related compounds as a chemo-preventive agent to control the incidence of cancers. The trials have had controversial results and some rejected the prophylactic role 29. On the other hand, in spite of a bunch of clinical and laboratory data, the mechanism of selenium activity is not fully determined for cancer treatment. One possible beneficial effect of selenium is related to its indirect effect on immune responses, as the consumption of selenium in a therapeutic regimen in addition to conventional anticancer treatments seems more efficient due to the immune stimulatory effect of this element. However, several researches support the direct anti-cancer effect of selenium as a result of anti-oxidant capacity of this trace element. However, like other trace elements, a major concern which limits selenium application is the toxicity.
Beside the anti-oxidant effect, SeNPs are also able to kill the cancer cells through the apoptosis mechanism. In fact, it has been already demonstrated that SeNPs can inhibit the tumor growth via induction of p53-mediated apoptosis 31. Moreover, in this study, SeNPs have been decorated with calcium sulfate which also may have some anti-cancer and apoptotic effects 32. Cytosolic calcium at high levels induces apoptosis via the mitochondrial pathway 33. Therefore, calcium sulfate is considered an apoptotic inducer that can trigger the intrinsic (Classical: mitochondrial) path-ways. Regarding these facts, CaSO4@SeNPs fabricated during this study can be considered as double-edged sword by which targeted cancer cells are faced to two powerful cancer killing agents.
In the past decade, with the growing attention to nanotechnology, SeNPs were highly research friendly elements especially because the biological properties of SeNPs are similar to Se ions even at lower doses with lower toxicity 14. One of the most probable applications of nanoparticles in the field of medicine is their possible capacity to be used in drug delivery systems although in the context of SeNPs, the story is somehow different. Like other metalloid NPs, SeNPs have been used in different modalities for cancer diagnosis and therapy. In fact, SeNPs have both capabilities to be used for targeting and also for killing cancer cells. Transferrin conjugated SeNPs as therapeutic agents loaded with doxorubicin showed a synergistic effect for cancer therapy with higher efficacy and fewer side effects 34. Likewise, hyaluronic acid decorated SeNPs as therapeutic agents demonstrated a higher tumor inhibition ratio and reduced tumor weight 35. These recent researches showed the potential of this nanoparticle to be used in drug delivery systems. On the other hand, the activation of apoptosis pathways is the mechanism that probably induces the direct anti-cancer effect of SeNPs. It is well documented that SeNPs induce apoptosis by depletion of mitochondrial membrane potential, Reactive Oxygen Species (ROS) overproduction, and cytochrome C release 34.
In addition to overproduction and accumulation of free radicals, selenium causes oxidative stress and/or endoplasmic reticulum stress and mediates cell survival by modulating Ca2+ release and consecutive apoptosis 36. In the present research, regarding the role of SeNPs in induction of apoptosis and also the similar role of calcium, the synergistic effect of these two pro-apoptotic agents has been demonstrated and results showed that the anti-cancer effect of CaSO4@SeNPs at the dose of 50 µg doubled in comparison to SeNPs alone. The same effect can also be observed at the doses of 25 µg and 10 µg. In fact, by these preliminary data, it can be proposed that CaSO4@SeNPs, in comparison to SeNPs, can act as a double-edged sword. However, the capacity of NPs for delivery of anti-cancer drug to their target cells should not be ignored 37. In other words, NPs not only have this ability to induce an anticancer response through the activation of apoptosis, but also are an appropriate candidate for targeting the cancer cells and are considered as a powerful delivery system which may accumulate more SeNPs in cancer cells in comparison with SeNPs that exclusively target the cancer cells.
However, interaction of selenium with intracellular proteins such as Glutathione peroxidase (Gpx), Superoxide Dismutase (SOD) or catalase, and any other enzymes that have cysteine in their active site and inhibition of their activity results in the accumul-ation of ROS in the cell cytoplasm 38. Therefore, considering the higher level of these enzymes in cancer cells due to higher level of metabolism and mitochond-rial respiration, an optimum dose of selenium may cause more toxicity for cancer cells compared to normal cells.
Conclusion :
To sum up, results of the present work demonstrate that the decoration of SeNPs as known cytotoxic NPs for breast cancer cells with calcium sulfate may increase their anti-cancer potential, which can be observed by lowering the effective dose. However, for discovering the exact corresponding mechanisms of this synergistic effect which are proposed to be related to activation of intrinsic apoptosis signaling and/or pH regulatory properties of CaSO4@SeNPs, further in vitro and in vivo studies are still needed.
Acknowledgement :
Research reported in this publication was supported by Elite Researcher Grant Committee under award number [963338] from the National Institute for Medical Research Development (NIMAD), Tehran, Iran.
Conflict of Interest :
The authors declare no conflicts of interest.
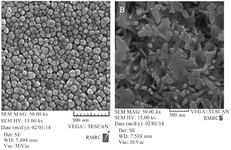
Figure 1. FSEM images of SeNPs, A) FESEM image of plain SeNPs showing the size of 60 nm. B) FESEM image of fabricated CaSO4@SeNPs demonstrating nanocomposites are rod shaped (400×100 nm).
|
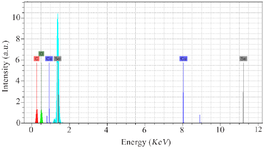
Figure 2. The EDS spectrum of SeNPs confirming the presence of Se atoms and the existence of SeNPs. Additional peaks of copper and carbon elements are attributed to the grid used for FESEM imaging.
|
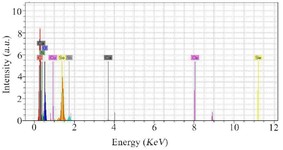
Figure 3. The illustration shows the presence of Se atoms and the existence of elemental NPs in the EDS spectrum of CaSO4@SeNPs. Additional peaks of copper, carbon, oxygen, and nitrogen elements are attributed to the grid used for FESEM imaging or calcium com-pound.
|
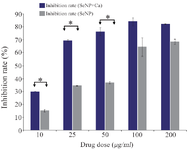
Figure 4. Comparison of cytotoxicity effect of CaSO4@SeNPs and SeNPs on 4T1 cells. The mean value of three repeats is presented and the standard deviation was negligible (<5%), yet the difference be-tween the inhibition rates obtained for SeNPs and CaSO4@SeNPs at concentrations of 10, 25 and 50 µg was statistically significant (p= 0.05).
|
|