Determining the Specific Activity of Anti-Rabies Sera and Immunoglobulin Using Atomic Force Microscopy of Cell Cultures
-
 Generalov, Sergey V
Russian Research Anti-Plague Institute Microbe, Saratov, Russia, Tel: + 7-8452-26-21-31; Email: svgeneraloff@gmail.com, rusrapi@microbe.ru
Generalov, Sergey V
Russian Research Anti-Plague Institute Microbe, Saratov, Russia, Tel: + 7-8452-26-21-31; Email: svgeneraloff@gmail.com, rusrapi@microbe.ru
-
Erokhin, Pavel S
-
Russian Research Anti-Plague Institute Microbe, Saratov, Russia
-
Abramova, Elena G
-
Russian Research Anti-Plague Institute Microbe, Saratov, Russia
-
Saratov State Vavilov Agrarian University, Saratov, Russia
-
Zhulidov, Ivan M
-
Russian Research Anti-Plague Institute Microbe, Saratov, Russia
-
Osina, Natalya A
-
Russian Research Anti-Plague Institute Microbe, Saratov, Russia
Abstract: Background: Mouse neutralization test is widely used to determine the level of anti-rabies antibodies, but it is labor-intensive and time consuming. Alternative methods for determining the neutralizing activity of anti-rabies sera and immunoglobulin in cell cultures are also known. Methods such as FAVN and RFFIT involve the use of fluorescent diagnostics. Determination of Cytopathic Effect (CPE) is often complicated due to features of rabies virus replication in cells. Atomic Force Microscopy (AFM) is able to detect the interaction of the virus with the cell at an early stage. Therefore, in this study, a method has been developed for determining the specific activity of anti-rabies sera and immunoglobulin using AFM of cell cultures.
Methods: The method is based on the preliminary interaction of rabies virus with samples of rabies sera or immunoglobulin drug, adding the specified reaction mixture to cell culture (Vero or BHK-21), and then measuring the surface roughness of the cells using AFM. AFM was carried out in the intermittent contact mode by the mismatch method in the semi-contact mode. The results were compared with the values obtained in the mouse neutralization test. The consistency of the results obtained by both methods was evaluated by Bland-Altman method.
Results: The increment in the surface roughness of the cells is a consequence of the damaging effect of the virus, which is weakened as a result of its neutralization by rabies antibodies. A dilution allowing 50% suppression of the increase in the surface roughness of cells was selected as the titer of rabies sera or immunoglobulin. In this case, the recommended range for determining the antibody titer is from 1:100 to 1:3000.
Conclusion: For the first time, a new methodological approach in virology and pharmaceutical research is presented in this study. The use of the proposed methodological technique will reduce the time from 21 to 2 days to obtain results in comparison with the mouse neutralization test; also, fewer laboratory animals are required in this approach which is in agreement with 3 R Principle.
Introduction :
Rabies is a dangerous zoonosis caused by the Rabies lyssavirus. Infection of people occurs through bites or saliva of infected animals and leads to fatal case if the measures for post-exposure prophylaxis are not adopted. Rabies vaccine and immunoglobulin are prescribed to prevent infection. The volume of injected anti-rabies immunoglobulin is associated with the specific neutralizing activity of the drug, which depends on the content of virus-neutralizing antibodies. The specific activity of rabies immunoglobulin starts at the dose of 150 IU per 1 ml according to the requirements of the World Health Organization (WHO) 1. In this case, passive immunity occurs from the moment of the bite to the start of the development of active immunity (7-8 days).
The specific activity of anti-rabies immunoglobulin is determined in the virus neutralization test on white mice 2. This method is based on the neutralization of rabies virus by dilutions of anti-rabies immunoglobulin and subsequent intracerebral administration of a mixture of virus-containing fluid and anti-rabies immunoglobulin to white mice. The test result is observed for 15-21 days while the number of animals survived and died from rabies is taken into account.
The virus neutralization test on white mice is a sensitive and specific method, yet labor-intensive. It requires a large number of animals, and a long observation period. In this regard, the tasks of developing and implementing alternative methods for detecting rabies virus and determining the specific activity of rabies drugs without the use of animals are important, which has been the main focus of WHO Expert Committee on Rabies as well 1.
Various in vitro methods have been developed to determine the level of anti-rabies antibodies and the detection of rabies virus. The Enzyme-Linked Immunosorbent Assay (ELISA) is one of such methods. Many researchers have noted the possibility of using ELISA to assess the level of anti-rabies antibodies in the blood serum of vaccinated animals and anti-rabies immunoglobulin preparations 3–5. ELISA results largely depend on the characteristics of the kit for the determination of antibodies. For example, an ELISA kit containing monoclonal antibodies against antigenic site III of the rabies virus glycoprotein allows the detection of neutralizing antibodies in anti-rabies immunoglobulin 4. Some reports note that ELISA measures all antibody types and does not discriminate the neutralizing antibodies; therefore, it is not reliable to predict the neutralizing activity of the anti-rabies sera or immunoglobulin 6-8. For this reason, laboratory techniques based on preliminary neutralization of the virus with antibodies remain relevant. Moreover, ELISA is not used as a pharmacopoeial method to determine the specific activity of anti-rabies immunoglobulin drugs.
The European Pharmacopoeia describes a method for determining the specific activity of an immunoglobulin preparation based on the use of fluorescence microscopy of infected cell cultures 9,10. Similar methods involve staining the cell monolayer with a conjugate of specific antibodies with a fluorescent label. Virus accumulation is assessed optically by detecting fluorescence groups of cells in which rabies virus antigens are accumulated.
There is an approach for detection of rabies virus without the use of fluorescent antibodies, based on visual observation of the Cytopathic Effect (CPE) by optical microscopy 11. Evaluation of the CPE is associated with the detection of destructive changes in individual cells and the cell monolayer. Therefore, the determination of the titer of the rabies virus by the CPE is especially complicated when a cell culture is observed in the late stages of cultivation, and cells of an intact culture are at the stage of dying. Moreover, some researchers note the absence of a typical cytopathic effect when the rabies virus is exposed to the cell 12. There are methods for detecting cytopathic changes of viral infection by analysis of stereological parameters using light microscopy 13,14. It should be outlined that the capabilities of optical devices are limited by the diffraction resolution limit. Therefore, using microscopy with higher resolution to study the interaction of the virus and the cell is preferable.
One of the methodologies for detecting the virus in cell cultures is the usage of Atomic Force Microscopy (AFM) 15,16. AFM methods are used to study the processes of interaction between viruses and both prokaryotic 17,18 and eukaryotic cells 19-24, detection of toxic proteins, and assessment of vaccine quality 25-27.
When studying the interaction of cells and a virus using AFM, the AFM intermittent contact mode is the most common scanning mode. This mode includes methods of semi-contact, mismatch and phase display. The semi-contact method is intended for investigating the topography of small-sized systems, including determining the linear dimensions of the research object (length, width, height), as well as the surface features of the preparation in terms of arithmetic mean and root-mean-square roughness of the cell wall surface. The mismatch method is commonly used to identify and characterize the fine ultra-structural details of an object. The phase display method is used to reveal the protein structures of biological objects by changing the phase of the cantilever shift.
The use of AFM makes it possible to detect the cytopathic effect of the virus in the early stages of its replication, such as cell size changes, virus adsorption, virus budding structures, and cell membrane rupture 22-24. It should be noted that the parameter characterizing the virus adsorption, budding, and membrane rupture is the roughness of the cell surface 23,24.
Previous studies have shown that cell size changes and cell membrane roughness depend on the infecting dose and the time of cultivation of rabies virus in cell culture 28. At the same time, the change in the roughness of the cell membrane being the result of the cytopathic effect of the virus seems to be the most indicative parameter for evaluating the activity of rabies virus and the level of anti-rabies antibodies that can neutralize the virus, thereby eliminating the effect of the roughness increment factor.
Thus, the objective of the study was developing methodological techniques for assessing the activity of substances containing rabies virus or anti-rabies antibodies using AFM of cell cultures
Materials and Methods :
Cell culture and rabies virus propagation: Attenuated strain of rabies virus "Moscow 3253" (GenBank: MF630920.1), adapted for replication on the transplanted Vero cell line, was used in this research. Continuous cell lines, Vero and BHK-21, were tested for the absence of mycoplasma and deployed for the investigation. Cells and viruses were cultured on MEM (Biolot, Russia) supplemented with 10% bovine serum (Biolot, Russia) at 37°С and 5% СО2. The state of the cell monolayer was evaluated by phase contrast microscopy using an inverted microscope.
Anti-rabies sera and immunoglobulin production: Anti-rabies immunoglobulin (Russian Research Anti-Plague Institute Microbe, Russia) was used in this study for post-exposure prophylaxis against rabies 29. Anti-rabies sera used for immunoglobulin were obtained using rivanol-alcohol fractionation from anti-rabies sera. Anti-rabies sera were collected from the blood of hyper-immunized horses.
Neutralizing activity of anti-rabies serum or immunoglobulin assay: Specific neutralizing activity of anti-rabies serum or immunoglobulin drug was determined in the mouse neutralization test according to the recommendations of the WHO 2 using the strain of the rabies virus (CVS). The results were calculated using the Reed and Muench method 2.
Determination of the activity of rabies virus and the neutralizing activity of anti-rabies serum and immunoglobulin using AFM
Growth of cell culture: A monolayer cell culture was preliminarily prepared. A sterile coverslip, the size of 18×18 mm, was placed in a well of a 6-well plate. Then, 2 ml of a suspension of Vero or BHK-21 cells was added to each well at a concentration of 104 cells/ml. Cells were incubated in a CO2 incubator (Sanyo, Japan) at 37°C for 24 hr. The cells formed a uniform dense monolayer at the end of the incubation period.
Sample preparation and virus neutralization: The anti-rabies immunoglobulin was diluted in a sterile MEM with a 5% bovine serum at a ratio of 1:50. A ratio of 1:5 was used in experiments with anti-rabies serum. Next, 1 ml of culture medium was added to sterile tubes. A working solution of serum or immunoglobulin was titrated with 0.5 ml in those tubes.
Then, an equal volume of rabies virus working solution in a similar growth medium was added to the tubes containing dilutions of the anti-rabies immunoglobulin, mixed and incubated in an incubator at 37°C. At the same time, tubes containing rabies virus working solution without adding rabies immunoglobulin, and also immunoglobulin working solution (1:50) or specific serum (1:5) without adding virus-containing fluid were incubated. For evaluating the activity of the rabies virus, it was prepared for breeding in a growth medium.
Adding samples to cells and incubation: The test samples were added to a prepared monolayer cell culture in the next stage of the study. Nutrient medium was removed from the wells of the plates containing coverslips with cell cultures before the introduction of the samples. The cell monolayer was washed once with DPBS and test samples were inoculated. Intact cells were left as negative control. The culture medium used in the experiment was added to them. The optimum final volume of added liquids was 2 ml. Sample plates were incubated at 37°C and 5% CO2 for 24 hr.
Fixation: The culture fluid was removed after incubation and the cell monolayer was washed with Dulbecco's solution. Then, 1 ml of 2.5% glutaraldehyde solution was added to the cells to fix the cell culture. Cell plates were incubated at 4±2°C for 24 hr. Then, the fixative solution was removed. The fixed cell monolayer was washed with 0.1 M phosphate-buffered saline and deionized water sequentially, and then dried in air at room temperature. AFM of cell cultures was performed after drying.
AFM assays: The Solver P47-PRO scanning probe microscope (NT-MDT, Russia) was used for AFM. The studies were carried out in the intermittent contact mode by the mismatch method in the semi-contact mode 15,26. The adhesion force was measured by force modulation in the continuous contact mode 15,17 using silicon tips of the NSG01 series (NT-MDT, Russia). The stiffness of the tips was 5.1 N/m or 0.1 N/m, the radius of curvature was 10 nm and the resonant frequency was 150 kHz or 120 kHz. The studies were carried out at the optimum values of the main scanning parameters. The amplitude of oscillations of cantilever (Resonance) was 22 units, the initial phase of its oscillations (Phase) -240°C, the scanning speed (Frequency) -0.75 Hz, the feedback gain of the feedback circuit (FB Gain) -0.3 units, and set point was 19 units. The value of set point and the initial level of DFL signal determined the magnitude of the force of interaction of the tip with the surface of the sample. The arithmetic average surface roughness of the cell membrane absolute error was determined by the roughness analysis using 30 values obtained in the experiment.
Interpretation of results: Image processing and analysis was performed using image analysis software (NT-MDT, Russia). The virus titer was the dilution value with the lowest virus concentration, which would cause a statistically significant increase in the surface roughness of the cells as compared to the control group. The titer of rabies antibodies was determined graphically. The values of reciprocal dilutions of the test sample were noted along the abscissa axis, and the index of inhibition of the increase in roughness was noted along the ordinate axis. This index was determined by the following formula and expressed as a percentage:
I=Np.k. -NNp.k.-Nn.k.×100%
where I is the roughness inhibition index, N is the average value of the surface roughness of cells treated with a mixture of immunoglobulin and virus, Np.k. is the average value of the roughness of cells treated with a working solution of the virus (Positive control), and Nn.k. is the average value of the roughness of uninfected intact cells (Negative control).
The titer of rabies antibodies in the test material was the value corresponding to the inhibition index of increase in roughness equal to 50%.
Statistical analysis: Statistical processing of the results was carried out using Nova software (NT-MDT, Russia) and Microsoft Excel (Microsoft, USA). The data were analyzed using test-test to compare differences between the groups. The consistency of the results for determining the activity of sera and immunoglobulin obtained by the proposed method and neutralization reaction in white mice was calculated by Bland-Altman method 30.
Results :
The optimum time interval was selected to determine the infectious activity of rabies virus against cell cultures at the first stage of the study. Dilutions of the fluid containing rabies virus were added into the cell monolayer and incubated for 1 to 48 hr. After that, changes in the cell membrane roughness were recorded (Figure 1 and Table 1). Changes in the adhesion force of cell cultures to the substrate surface were also recorded at this stage. These results were used for further studies to investigate the activity of rabies sera and antibodies.
Virus titer values obtained using AFM were assessed in experiments to determine the specific activity of rabies serum and immunoglobulin in the preparation of working dilution. The value of the working dilution exceeded the titer of the virus from 100 to 1000 times. The interaction of the virus with the studied samples of serum or immunoglobulin occurred at 37°C for 60±15 min. The specific activity of the rabies immunoglobulin sample is shown in figure 2. The reciprocal antibody titer of the test sample corresponded to 2.75 lg in experiments using Vero cell culture and 3.11 lg in experiments using BHK-21 cell culture. Thus, the choice of the cell line did not have a significant effect on the result of the study.
The obtained values of specific activity were compared to similar parameters obtained in experiments using the virus neutralization test on white mice 2. The results of a comparative study of samples of rabies serum and immunoglobulin are presented in figure 3.
Discussion :
Changes in cell surface assay under the influence of the rabies virus: The results of the AFM cell cultures indicate an increase in roughness in the first hours of interaction of the cell with the virus (Table 1). An increase in roughness in the first hours after infection may be associated with the adsorption of the virus and its penetration into the cell. The rise in roughness after 24 hr can be explained by the release of virus particles from the cell. Similar results were obtained for other viruses. Atomic force microscopy of the HTLV-1 virus on MT-2 cell culture recommends recording the virus budding after 24 hr 22. The picornavirus causes an increase in the roughness of infected BHK-21 when cultured for more than 4 hr 23. An increase in the roughness of the cell membrane was demonstrated when baculovirus was cultured on Sf9 cells for more than 24 hr 24. In this study, the 24 hr observation period was sufficient to detect changes in the ultrastructure of the cell surface (Figure 1) caused by small dilutions of the rabies virus. This circumstance makes it possible to determine the titer of vaccine liquid. An increase in adhesion power additionally indicates changes in the ultrastructure of the cell surface, an increase in roughness and the number of areas of contact with the surface. However, this parameter is not indicative of virus activity.
Rabies antibodies titer: The specific activity of anti-rabies sera or immunoglobulin is associated with the anti-rabies neutralizing antibody level. A methodical approach based on preliminary neutralization of a known dilution of the rabies virus with dilutions of substances containing anti-rabies antibodies is usually used to determine the antibody level. The titer of the residual virus is determined in mice or cell cultures 2,9. In this work, a similar approach to neutralizing the virus was used to determine the activity of anti-rabies sera or immunoglobulin. The specific feature of the proposed methodological approach is the further residual virus assay using AFM method, as described in the previous section.
It should be assumed that the addition of antibodies to the rabies virus inhibits the increase in roughness through neutralizing the virus. Accordingly, the inhibition of the increase in roughness will differ depending on the added dilution of antibodies to the same dilutions of the virus. The roughness values of infected cells and cells treated with a mixture containing anti-rabies antibodies and rabies virus were compared with the roughness values of infected and intact cells. The coefficient value of inhibition of the increase in roughness was determined on the basis of the results obtained at the second stage of the study and was calculated for each dilution of anti-rabies sera or immunoglobulin according to the formula mentioned in Materials and Methods section. The dependence graph of the inhibition of the increase in roughness and the titer of anti-rabies sera or immunoglobulin was developed according to these values. A sample of the graph is shown in figure 2. In this graph, the point at which roughness inhibition reaches 50% was marked. For the indicated value, the corresponding titer of rabies serum or immunoglobulin was detected on the graph. The indicated titer value was taken as the activity value. It should be noted that the recommended range for determining the antibody titer using the proposed approach was from 1:100 to 1:3000.
Comparison of the proposed method with the mouse neutralization test: The neutralization reaction of the rabies virus in white mice is the "gold standard" in determining the activity of anti-rabies serum preparations. This method is used in the production of anti-rabies drugs. For this reason, the method was applied to confirm the results of the study about the activity of anti-rabies serum and immunoglobulin samples obtained using the proposed method.
Comparison of the research results obtained by the two indicated methods was carried out according to the Bland-Altman method (Figure 3). The Bland-Altman plots displayed the difference between the logarithm values of antibody titers obtained by the two compared methods (along the y-axis) relative to the average of these values (along the x-axis). The mean difference in values and the limits of agreement (Means±SD of 1.96) was calculated. The average difference characterizes the systematic discrepancy between the results, and the limits of agreement characterize the range of values. Bland-Altman analysis shows that systematic discrepancy between the results obtained by the two methods was within the boundaries of the 95% confidence interval, which indicates the consistency of both methods. Therefore, the proposed methodological approach can be used in virology and pharmaceutical research.
Conclusion :
For the first time, a methodological approach is presented for determining the activity of the rabies virus and the titer of anti-rabies antibodies in sera and immunoglobulin, based on the study of changes in the ultrastructure of the cell surface by AFM.
This method will reduce the required time from 21 to 2 days to obtain the results in comparison with the mouse neutralization test. Moreover, it does not require the use of diagnostic antibody conjugates. Deployment of AFM will decrease the likelihood of errors that occur when assessing the damaging effects of rabies virus on cell cultures. Both cell cultures, Vero and BHK-21, can be used to analyze the specific activity of rabies sera and immunoglobulin using the proposed method. The technique using AFM is proposed for application in virology and pharmaceutical research and it minimizes the number of laboratory animals required in experiments, which is in line with the 3 R Principle 31.
Acknowledgement :
There was no financial support in this study. None of the authors have any conflict of interest to declare.
Ethical Approval :
Authors are aware of, and comply with best practice in publication ethics specifically with regard to authorship (Avoidance of guest authorship), dual submission, manipulation of figures, competing interests and compliance with policies on research ethics. Authors adhere to publication requirements that submitted work is original and has not been published elsewhere in any languages. The studies described in this article were carried out at Russian Research Anti-Plague Institute Microbe.

Figure 1. AFM visualization of the surface ultrastructure of cell cultures after 24 hr of cultivation (Image obtained by the mismatch method; section profile; image obtained by the semi-contact method): A) intact cell culture- BHK-21; B) rabies virus infected cell culture-BHK-21; C) intact cell culture- Vero; D) -rabies virus infected cell culture- Vero.
|
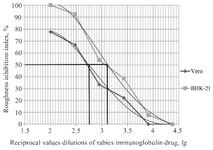
Figure 2. Titer of specific activity of rabies immunoglobulin on cell cultures of Vero and BHK-21.
|
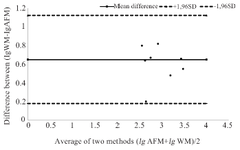
Figure 3. Comparison between the consistency of the activity values of antirabies sera and immunoglobulin obtained by Atomic Force Microscopy (AFM) and neutralization reaction in White Mice (WM).
|
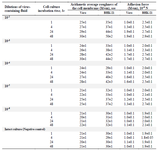
Table 1. The rabies virus effect on the surface roughness of Vero and BHK-21 cells
Note: Each parameter was determined from 3 to 5 experiments.
|
|