The Effect of Sodium Selenite on Expression of Mitochondrial Transcription Factor A during In Vitro Maturation of Mouse Oocyte
-
Moshaashaee, Tina
-
Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
Zavareh, Saeed
-
Faculty of Biology, Damghan University, Damghan, Iran
-
Pourbeiranvand, Shahram
-
Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
 Salehnia, Mojdeh
Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, Tel: +98 21 82880(3562), E-mail: salehnim@modares.ac.ir, mogdeh@dr.com
Salehnia, Mojdeh
Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran, Tel: +98 21 82880(3562), E-mail: salehnim@modares.ac.ir, mogdeh@dr.com
Abstract: Background: The aim of the present study was to investigate the effect of Sodium Selenite (SS) supplemented media on oocyte maturation, expression of mitochondrial transcription factor A (TFAM) and embryo quality.
Methods: Mouse Germinal Vesicle (GV) oocytes were collected after administration of Pregnant Mare Serum Gonadotropin (PMSG); in experimental group 1, oocytes were cultured and then subjected for in vitro maturation in the absence of SS, and in experimental group 2, they were matured in vitro in the presence of 10 ng/ml of SS up to 16 hr. The control group included MII oocytes obtained from the fallopian tubes after ovarian stimulation with PMSG, followed by human chorionic gonadotropin. Then, the expression of TFAM in MII oocytes in all three groups was investigated using real-time RT-PCR. The fertilization and embryo developmental rates were assessed, and finally the quality of the blastocysts was evaluated using propidium iodide staining.
Results: The oocyte maturation rate to MII stage in SS treated group was significantly higher than non-treated oocytes (75.65 vs. 68.17%, p<0.05). Also, the rates of fertilization, embryo development to blastocyst stage as well as the cell number of blastocyst in SS supplemented group were higher than other experimental group (p<0.05). There was a significant decrease in TFAM gene expression in both in vitro groups compared to the group with in vivo obtained oocytes (p<0.05). Moreover, there was a significant increase in TFAM gene expression in oocytes that matured in the presence of SS compared to that of the group without SS (p<0.05).
Conclusion: Supplementation of oocyte maturation culture media with SS improved the development rate of oocytes and embryo and also enhanced TFAM expression in MII oocytes which can affect the mitochondrial biogenesis of oocytes.
Introduction :
In Vitro Maturation (IVM) of oocyte refers to development of immature germinal oocytes in the medium supplemented with some growth factors and hormones 1. The numerous successful pregnancies following IVM have caused this therapeutic method to be considered low risk and effective in assisted reproduction 1-5. There are several groups of candidates for this method such as patients with polycystic ovarian syndrome, low follicles reserves, repeated IVF failure, ovarian hypertension, resistant ovary syndrome and young women who suffer from different types of cancer 1-5. Although the rate of pregnancy after IVM of oocytes increases in these patients, it is still considered as an experimental method 5. It was reported that various factors such as the culture media, supplementation of hormones, co-culture with somatic cells and adding some antioxidants could affect the outcom of oocyte IVM 6-9.
For oocyte maturation, the mitochondria are required as the source of energy and for intracellular calcium storage 10,11. It also regulates calcium fluctuations during fertilization which is very important for the fertilization and embryonic development 11. The transcription of mitochondrial DNA is essential for its biogenesis during the oocyte development and mitochondrial DNA (mtDNA) content is correlated with oocyte fertilization potentional 12-15 and immature oocytes have fewer copies of mtDNA 13. Mitochondrial transcription factor A (TFAM) is an important mitochondrial DNA transcription factor and is coded by nuclear DNA 16. It creates structural stability and converts the genome to nucleotide-like structures by shortening the length of the mitochondrial genome through covering it and gives a regular curve to it 16,17. Decline in the TFAM expression is associated with the decrease in mtDNA content; in other words, the mtDNA content is directly related to the expression of TFAM 18. Moreover, the high level of Reactive Oxygen Species (ROS) causes damage to mtDNA 19-22.
It has also been shown that Sodium Selenite (SS) as a non-enzymatic antioxidant improves development of follicles, oocyte and embryo by elevating the total antioxidant capacity and reducing the level of ROS 13,23-26. In our previous studies, it was shown that adding SS to the oocyte maturation media increased mtDNA content of the oocytes 13,26. This study was designed to bring some additional information regarding the effect of SS on the biogenesis of oocyte mitochondria through TFAM expression. Thus, the aim of the present study was to investigate the effect of SS supplementation in the culture media of immature oocyte on TFAM expression as well as oocyte and embryo development.
Materials and Methods :
All laboratory materials, except those mentioned, were purchased from Sigma Aldrich, England.
Animal model: In this study, 60 female NMRI mice, 6-8 weeks old and 10 male NMRI mice, 8-12 weeks old were used. The mice were kept under standard conditions of 12 hr light: 12 hr dark, 20-24°C, and 40-50% humidity in the animal house of Tarbiat Modares University. The mice had adequate access to food and water.
Study design: For investigating the effect of sodium selenite on TFAM expression of the oocyte, the cumulus cells were removed from oocytes in all study groups. The three groups in this study were (a) in vivo control group in which the metaphase II (MII) oocytes were collected from fallopian tubes, (b) experimental group 1 in which in vitro maturation of Germinal Vesicle (GV) oocytes was done in the absence of SS, and (c) experimental group 2 in which in vitro maturation of GV oocytes was done in the presence of SS.
In both in vitro matured groups, the oocyte maturation and embryonic developmental rates and blastocyst cell count were studied in comparison with in vivo obtained blastocysts. Also, TFAM expression in in vitro obtained MII oocytes was analyzed and compared with in vivo obtained MII oocytes. All experiments were approved by the ethics committee of Tarbiat Modares University, Tehran, Iran (IR.MODARES.REC.1398. 006).
GV oocytes collection: In order to collect the GV oocytes, 10 IU of Pregnant Mare Serum Gonadotropin (PMSG) were injected to each mouse intraperitoneally and 48 hr later, the mentioned mice were sacrificed through cervical dislocation. Initially, by cutting the skin and then through a longitudinal incision in the abdominal region, two ovaries were extracted and placed within the culture media. Then, the oocytes at GV stage were isolated using the needle attached to the insulin syringe under a stereo microscope. The GV oocytes with a centrally located nucleus, clear cytoplasm, uniform zona pellucida and homogeneous perivitelline space were chosen 13. The cumulus cells were detached using repeated pipetting and enzymatic methods. All GV oocytes were denuded and were considered for in vitro maturation in two experimental groups.
MII oocytes collection: To collect the MII oocytes, 10 IU of PMSG were injected intraperitoneally to adult female mice and 48 hr later, 10 IU of human Chorionic Gonadotropin (hCG) were also injected. Thereafter, 16 hr after the last injection, the mice were sacrificed through cervical dislocation and MII oocytes were collected from their fallopian tube, denuded, and then kept at -80°C for molecular study.
In vitro maturation: The collected GV oocytes were cultured in α-MEM medium supplemented with 50 of μg/ml penicillin, 75 of μg/ml streptomycin, 0.23 mM of sodium pyruvate, 10% of fetal bovine serum, 75 mIU/ml of recombinant follicle stimulating hormone, 10 IU/ml of hCG and in the presence and absence of 10 ng/ml of SS according to the experimental design under mineral oil at 37°C, 100% humidity in 5% CO2 for 16 hr 13. The maturation of oocytes was assessed under inverted microscope. The oocytes with clear nucleus were categorized as GV oocytes; those with uniform cytoplasm and without nucleus were classified as Germinal Vesicle Breakdown (GVBD) or metaphase I, and the oocytes with first polar body were considered as the MII oocytes. This experiment was replicated at least seven times. MII oocytes were collected and considered for the subsequent studies.
In vitro fertilization: The sperm was prepared from adult male mice at the age of 8-12 weeks. The mice were sacrificed through cervical dislocation and their epididymis tail was separated and transferred to 300 µl drops of culture medium under mineral oil. Using a needle attached to insulin syringe, several small cuts were made in the epididymis tail so that the sperm was expelled out of the epididymis and kept for 1.5 hr in incubator at 37°C, 5% CO2 and 100% humidity until the completion of capacitation. The oocytes were transferred to drops containing a concentration of 105 sperm per ml. They were then kept under the following conditions for 4-6 hr and then, the oocytes were washed several times with fresh media and culture for 120 hr. During the culture period, the embryonic developmental stages including the two-cell, four-cell, morula and blastocyst were assessed using inverted microscope. The embryos at blastocyst stage were collected for staining with Propidium Iodide (PI).
Harvesting of in vivo blastocyst: In order to collect in vivo obtained blastocysts, female mice were injected with 10 IU of PMSG hormone followed by 10 IU of hCG after 48 hr. Then, they were placed alongside a male mouse. The morning of the next day, the pregnant mice were separated and 96 hr after hCG injection, the pregnant female mice were sacrificed through cervical dislocation and the embryos were collected by flushing uterine horns.
Blastocysts staining: For embryo staining, the collected blastocysts from both in vitro groups (n=3 in each group in 3 repeats) were put in100 µg/ml of PI and 1% of Triton ×100 for 10 s. Then, the stained embryos were placed in glycerol and were investigated under fluorescence microscope 27.
Molecular analysis: In order to investigate the expression of TFAM gene in MII oocytes, real-time RT-PCR was used. Total RNA was extracted from the MII oocytes (n=10 in each group) using the TRIzol ® reagent (Invitrogen, USA) according to the manufacturer’s guidance. The quality of RNA was evaluated using the spectrophotometry (Eppendorf, Hamburg, Germany). The ratio of optical density, A260/A280≥1.8, was accepted for RNA and used for reverse transcription. The cDNA synthesis was performed based on the cDNA synthesis kit (Takara, Japan) manual. The sequence of forward and reverse primers (Table 1) was designed using Allele ID software, version 7.5 (PREMIER Biosoft, USA). To ensure specificity for the mRNA of target genes and nonreplication of genomic DNA, the sequence of primers was blasted using Primer 3 software at NCBI website. In addition, no genomic DNA replication by each of the designed primers was confirmed through a real-time RT-PCR reaction with 25 ng of total RNA solution as template and electrophoresis of PCR product on 1.5% agarose gel. In this reaction, 25 ng of cDNA synthesized for each gene was used as positive control in a separate microtube in qPCR reaction. The real-time RT-PCR reaction was performed by StepOnePlus™ Real-Time PCR System (Applied Biosystems, USA) and using SYBR Green qPCR Master Mix (Ampliqon, Denmark). The mixture of the reaction contained SYBER Green (5 µl), forward primer (0.5 µl), reverse primer (0.5 µl), and cDNA (4 µl) in 10 µl of final volume. PCR reaction was adjusted for 15 min at 95°C. It was followed by 40 cycles of denaturation at 95°C for 10 s and annealing/extension at 60°C for 45 s. The level of expression of each gene was normalized through the β-actin as internal control gene and then relative expression of genes was calculated using the -2-∆∆Ct formula.
Statistical analysis: The statistical analysis was performed using SPSS® version 24 (SPSS Inc., Chicago, USA). After investigating the normal distribution of data using Shapiro-Wilk test, in order to compare the percentage of GV, GVBD, and MII oocytes as well as viability between two experimental groups, unpaired t-test was used. The real-time RT-PCR data were normalized using Livak method and one-way analysis of variance (ANOVA) and post hoc Tukey tests were used. The results have been presented based on Mean±SD and p≤0.05 was considered statistically significant.
Results :
Developmental and fertilization rates: As shown in table 2, after 16 hr of in vitro culture of GV oocytes, the mean total percentage of survived oocytes in the group treated with SS and in the group matured without SS supplementation was 90.97±4.69 and 87.82±5.75, respectively and there was no significant difference between the two studied groups in this regard. Further, the rate of MII oocytes in the SS treated group (75.65±6.56) was significantly higher than the group matured without SS (68.17±2.47; p<0.05). Statistical analysis indicated that the rates of GV and GVBD oocytes did not have any significant difference between the two tested groups.
The mean percentage of fertilization, two-cell, four-cell, and morula embryos in all studied groups are summarized in table 3. The mean percentage of oocytes that fertilized and reached to blastocyst stages in the presence of SS was 81.39±3.51 and 17.14±10, respectively, while those of group without SS treatment were 70±1.52 and 10.71±10, respectively. According to statistical analysis, the rates of fertilization and embryo development in the groups undergoing SS treatment increased significantly compared to the group without it (p<0.05).
Blastocyst cell count: The representative figures of blastocysts obtained from in vitro matured oocytes in the absence and presence of SS and in vivo obtained blastocysts as controls that were stained with PI are demonstrated in figure 1 A-C. As these figures show, the cell number of blastocyst was significantly higher in SS treated group in comparison with the group matured without SS (61±0.81 vs. 38±0.81; p<0.05). The in vivo obtained blastocysts significantly showed higher cell number (80.25±0.83) in comparison with both in vitro matured blastocysts (p<0.05; Figure 1D).
Real-time RT-PCR: The relative expression of TFAM to housekeeping gene (β-actin) in the oocytes obtained in vivo and in vitro in the presence and absence of SS was1.02±0.03, 0.7±0.04, and 0.25±0.07, respectively. The expression levels of TFAM decreased significantly in both IVM culture groups in comparison with in vivo obtained oocytes (p<0.05; Figure 2). Comparing the two in vitro groups, relative expression of TFAM was significantly higher in the oocytes undergoing SS treatment compared to the group without it (p<0.05; Figure 2). The representative figure of PCR product in the studied groups is demonstrated in figure 2B.
Discussion :
The present study investigated the effect of SS on the maturation of GV oocytes as well as the expression of TFAM in in vitro cultured oocytes in comparison with MII oocytes obtained from fallopian tubes.
The results of the present study are in line with previous studies that showed SS can be considered as an effective factor in the oocyte maturation and the rates of developmental parameters of in vitro cultured oocytes treated with SS increased significantly compared to the oocytes cultured in the medium without SS 13,25. The similar effects of SS supplement in the culture medium on the development of follicle and oocyte in some mammal species have also been previously reported 23,26,28-31. The obtained results may be related to the antioxidant properties of SS by reducing the ROS 13,26,28-31. A research has also shown that the reduction in the level of ROS occurred through the antioxidant activity of SS in the oocyte maturation medium 26. Also Uhm et al demonstrated that adding SS to swine embryo culture medium caused reduction of ROS and glutathione peroxide level was elevated 30. Recently, Xiong et al observed that the activity of glutathione peroxidase of oocytes and DNA integrity of cumulus cells increased significantly in the presence of SS during IVM of oocyte 23. In contrast to our results, Lizarraga et al, demonstrated that adequate SS concentrations added during IVM of bovine oocytes increased viability and non-apoptotic cumulus cells 24. Moreover, they revealed that neither nuclear maturation nor the developmental capacity of oocytes was modified by SS supplementation during IVM 24. These differences with the results of our study may be related to the use of denuded oocytes in our study instead of cumulus oocyte complexes or different types of mammalian species.
Our results also showed the same positive effect of SS on the developmental rates of embryo to blastocyst stage. It is suggested that the SS improved the embryo quality by alteration in the ROS level during embryo culture. It was shown that adding 10 ng/ml of SS to the culture medium of bovine oocyte improved the embryo quality and caused an increase in hatching rate of embryos 24. Also SS may affect embryo development through intracellular signaling by activation of phosphoinositide 3-kinase/Protein kinase B (PKB), Akt (PI3K/AKT), and extracellular signal-regulated kinases 31.
Another factor contributing to the effect of SS on oocytes development is reduction of oocyte apoptosis. Some studies have shown positive effects of SS on the inhibition of apoptosis pathway in other cell types 32-34. Our molecular analysis in this study indicated that in both IVM groups, TFAM gene expression of MII oocytes had decreased in comparison with in vivo obtained oocytes. This suggests the effect of culture medium conditions on the incidence of TFAM gene expression, which could influence the mitochondrial biogenesis of oocytes during culture period. On the other hand, previous studies have shown a direct relationship between diminished mtDNA levels and reduced number of mitochondria in poor quality oocytes 13. Thus, the reduction of expression of TFAM in both in vitro groups can be synonymous to decreased development of these oocytes. In this regard, Ghorbanmehr et al also showed that the obtained mitochondrial DNA (mtDNA) copy number of oocytes maturated in the culture medium in comparison to the oocytes obtained from in vivo conditions can be due to the poor quality of these oocytes; moreover, in the oocyte treated with SS, this parameter (mtDNA) was improved and reached to the same value similar to in vivo group 13. The SS may exert its effects through affecting the nuclear respiratory factor 1 as the main regulator of TFAM gene expression. For example, in hepatic cells of mice, it has been shown that the overexpression of NRF-1 leads to an increase in the expression of TFAM and mtDNA content 35. Another possibility is that the SS can indirectly influence the expression of TFAM by affecting the incidence of other factors such as miRNAs 36. To prove these suggestions, more studies are required.
Conclusion :
In conclusion, supplementation of oocyte maturation culture media with SS improved the development rate of oocytes and embryo and also enhanced TFAM gene expression in MII oocytes that could affect the mitochondrial biogenesis of oocytes.
Acknowledgement :
This work was supported by Tarbiat Modares University of Medical Sciences as MSc thesis. The authors clarify there is no any conflict of interest.
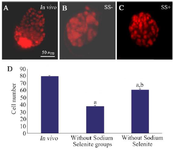
Figure 1. Staining of blastocysts derived from in vivo condition (A) and in vitro matured germinal vesicle oocytes in the absence (SS-; B) and presence of Sodium Selenite (SS+; C). The comparison of total cell number of blastocysts in previous groups was shown in part D. Letter a indicated the differences of each group with the control group and letter b showed significant differences between Sodium Selenite treated group with non-treated group (p<0.05).
|
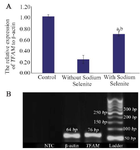
Figure 2. The relative expression of TFAM and β-actin in MII oocytes obtained from in vivo and in vitro conditions in the presence and absence of Sodium Selenite (A). a: significant differences with in vivo group; b: significant differences with non-treated in vitro group (p<0.05). The representative figure of gel electrophoresis of PCR product in studied groups (B). No template control (NTC).
|

|
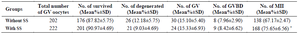
Table 2. Developmental rates of in vitro cultured GV oocytes in the Sodium Selenite (SS) treated and non treated groups
* Significant differences compared with other group in the same column (p<0.05). Seven experimental replicates were performed for each group.
GV= Germinal Vesicle, GVBD=Germinal Vesicle Breakdown.
|

Table 3. Fertilization and embryonic developmental rates of oocytes treated with Sodium Selenite (SS) treated and non treated groups
* Significant differences compared with other group in the same column (p<0.05).
|
|