Evaluation of Metastasis Suppressor Genes Expression and In Vitro Anti-Cancer Effects of Zinc Oxide Nanoparticles in Human Breast Cancer Cell Lines MCF-7 and T47D
-
Sharifian, Faryad
-
Department of Genetics and Biotechnology, Faculty of Biological Sciences, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran
-
Behboodi, Sorayya
-
Department of Biology, Tehran Shargh (East), Payam Noor University, Tehran, Iran
-
Ghodratpour, Fatemeh
-
Department of Genetics and Biotechnology, Faculty of Biological Sciences, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran
-
 Baghbani-Arani, Fahimeh
Department of Genetics and Biotechnology, Faculty of Biological Sciences, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran, Tel: +98 21 36725011, E-mail: Baghbani.f@gmail.com
Baghbani-Arani, Fahimeh
Department of Genetics and Biotechnology, Faculty of Biological Sciences, Varamin-Pishva Branch, Islamic Azad University, Varamin, Iran, Tel: +98 21 36725011, E-mail: Baghbani.f@gmail.com
Abstract: Background: Metallic nanoparticles are useful materials to be applied in biomedical research. In this study, the possible apoptotic and anti-metastatic activity of Zinc Oxide Nanoparticles (ZnONPs) was assessed in breast cancer cells.
Methods: First, in vitro cell viability was investigated by MTT assay in two human breast cancer cells (MCF-7 and T47D) and normal Human Embryonic Kidney (HEK293) cells at 37°C overnight. Apoptosis induced by ZnONPs was evaluated by annexin V/PI staining, cell cycle analysis and caspase assay in cancerous cells. Moreover, quantitative real-time PCR was employed for the detection of two metastasis suppressor genes (KAI-1 and NM23) expression in cancerous cells.
Results: Data demonstrated that ZnONPs exert a dose-dependent inhibitory effect on the viability of T47D and MCF-7 cells, while no cytotoxic effect was observed on normal HEK293 cells. The mRNA expression levels of KAI-1 and non-metastatic protein (NM23) genes were up-regulated in ZnONP-exposed cancerous cells. ZnONPs were also found to enhance the apoptosis properties of cells by annexin V/PI staining, and caspase assay in cancerous cells. Furthermore, ZnONPs can increase sub-G1 population as compared to negative control.
Conclusion: Our findings showed that ZnONPs induce apoptotic activity and can modulate metastasis by up-regulating of KAI-1 and NM23 gene expression in two breast cancer (MCF-7 and T47D) cells.
Introduction :
Breast cancer is the second most frequent malignant tumor in women, with 1.62 million new cases reported in 2012 1,2. Although conventional therapeutic options such as surgery, chemotherapy and radiation therapy have been recommended for treatment of breast cancer, all these approaches suffer from major drawbacks 3. In this regard, nanotechnology has provided considerable advancement as a promising area in medicine, drug delivery and anti-cancer applications 4.
The Zinc Oxide Nanoparticle (ZnONP) has been increasingly explored for biomedical applications, particularly due to its anti-cancer properties 5. Moreover, ZnONPs have been investigated for a wide variety of applications like functioning as nanosensors, energy storage materials, nano-electronic/ nano-optical devices, cosmetic products, etc 6,7. ZnONPs are being used in a broad spectrum such as gene/drug delivery and cancer therapy, with a focus on cell cytotoxicity induction at in vitro and in vivo levels 4,8.
Recently, several studies have suggested the cytotoxic effects of ZnONPs against several types of cancers. Wahab et al reported that about 13±2 nm ZnO nanoparticles induced significant apoptosis in HepG2 cells and human MCF-7 cells, with reactive oxygen species and oxidative stress being the major component in cytotoxicity 9. As more knowledge is obtained regarding alteration of gene expression during tumor progression, more potential methods are suggested for inhibiting metastatic cascades.
In this way, Metastasis Suppressor Genes (MSGs) have frequently been identified for the regulation of various cellular responses like cell invasion, tumor progression, and programmed cell death 10,11.
Among these, the most well-known research suggesting down-regulated NM23 gene expressions is related to several types of high metastatic potential cancer cells.
Also, different studies have indicated that KAI1 inhibits metastasis of melanoma and breast cell lines 12,13. Regarding its role as a metastasis suppressor, down regulation of KAI-1 gene expression is mostly demonstrated during ovarian and prostate cancers 14,15.
The current study investigated the apoptotic effects of ZnONPs via caspase assay, annexin V-FITC/PI staining, and cell cycle analysis against two human breast cancer cell lines. Also, real-time PCR was used to measure the expression of two metastasis suppressor genes (KAI-1 and NM23).
Materials and Methods :
Preparation of ZnONPs: ZnONPs with an average size of 10 to 30 nm were supplied by US Research Nanomaterials, Inc. (Twig LeafLane, Houston, USA). The size and morphological characteristics of ZnONPs were determined by transmission electron microscope (Zeiss-EM10C-100 KV, Germany).
Cell culture: A Human Embryonic Kidney (HEK293) and two breast cancer (MCF-7, T47D) cells were purchased from the National Cell Bank, Pasteur Institute of Iran. The cell lines were grown in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% (v/v) FBS in CO2 incubator at 37°C.
MTT assay: To reveal the dose-dependent cytotoxic effects of ZnONPs, MTT assay was used. T47D, MCF-7 and HEK293 cell lines were seeded (Density rang of 1×104 cells per well) in 96-well micro-plates containing DMEM medium, and allowed to attach for 24 hr. After overnight incubation, the cells were treated with various concentrations of ZnONPs ranging from 1, 10, 100, to 1000 µg/ml for overnight. After the exposure was done, the MTT dye (5 mg/ml) solution was added to each well for four hr. For dissolving the MTT formazan products, the culture medium was removed from each well followed by transferring equal amount of Dimethyl Sulfoxide (DMSO). The cells were incubated with DMSO at 37°C for 30 min and absorption was calculated at 570 nm.
Analysis of KAI-1 and NM23 gene expression: The T47D and MCF-7 cell lines at a density of 5×104 cells/ml were cultured into six-well plates followed by incubation at 37ºC in CO2 incubator. After 24 hr of incubation, the cells were treated with ZnONPs for another 24 hr. Total RNA isolation from the T47D and MCF-7 cell lines was conducted using the EasyPure® RNA Kit (TransGen Biotech ER101-01, China) according to the manufacturer’s protocol. The cDNA synthesis was done using the EasyScript® First-Strand cDNA Synthesis Kit (TransGen Biotech AE301-02, China) based on the manufacturer’s protocol. The sequence of forward and reverse specific primers for glyceraldehyde-3-phosphate dehydrogenase (GAPDH), KAI-1, and NM23 genes was designed using Primer Express Software version 3.0 (Table 1) and verified using BLAST analysis at NCBI (www.ncbi.nlm. nih.gov/blast). The GAPDH was used as the housekeeping gene for normalization within a given sample. The quantitative real-time polymerase chain reaction was performed in ABI7300 real-time PCR system (Applied Biosystems Company, USA) with SYBR GREEN® (Non-specific DNA-binding factors) based on the manufacturer’s protocol. The relative quantifications of NM23 and KAI-1 mRNA expression levels were compared with the housekeeping gene (GAPDH) using the 2–ΔΔCt method 16.
Caspase assay: In vitro quantitative determination of caspase 8 and 9 proteolytic enzyme activities were evaluated in the MCF-7 and T47D cells (3-5×105 cells per sample) based on the procedure of ApoTarget™ Caspase Colorimetric Protease Assay (Invitrogen) kit. Lysis of the cells was performed using caspase assay buffer and cells were incubated with the caspase 8 substrate (IETD-pNA) and the caspase 9 substrate (IEHD-pNA) for two hr at 37°C in a dark room. The absorbance of both treated and untreated samples was calculated at 400 or 405 nm. Fold-increase in caspase 8 and 9 activities of treated cells was evaluated by comparison with the level in the untreated control group.
Annexin V-FITC /PI staining: ZnONPs-induced apoptosis in cells was performed using annexin V-FITC and PI double staining detection kit (Hoffman-La Roch Ltd., Basel, Switzerland) via flow cytometry. The MCF-7 and T47D cells (1×106) were treated with ZnONPs in a six-well plate, for overnight incubation followed by twice washing with cold PBS and centrifugation at 3,000 rpm for 5 min. Thereafter, 500 ml of binding buffer were added to the cells. Then, the cells were stained in annexin V-FITC and PI and incubated at 25°C for 15 min in darkness. Finally, the percentages of apoptosis and necrosis of treated and untreated cells were determined by a flow cytometer (Cyflow, UK).
Cell cycle analysis: T47D and MCF-7 cells were treated with IC50 concentration of ZnONPs. The two cells were harvested by trypsin and washed with PBS, followed by fixation in ice-cold 70% ethanol at 4°C for 30 min. The cells were washed in PBS twice and treated with 100 µg/ml of RNase, and then stained with propidium iodide for 1 hr at 25°C in the dark.
Statistical analysis: All data were statistically analyzed using SPSS software version 22. The values were represented as the mean±standard deviation and differences between test and control were evaluated using one-way ANOVA.
Results and Discussion :
TEM images: The size and morphological characteristics of ZnONPs were determined by Transmission Electron Microscope (TEM) and it was revealed that ZnONPs images were spherical in shape (Figure 1).
Cytotoxicity results: The ZnONPs’ effects on the cell viability of T47D, MCF-7 and HEK293 cells were assessed using the MTT assay. The cancer and normal cells were exposed to ZnONPs for 24 hr at dose levels of 1-1000 µg/ml. The data showed that ZnONPs up to the maximum concentration of 1 μg/ml did not significantly increase the toxicity of cancer cells. In the case of MCF-7 cells, after treatment with various concentrations of ZnONPs, a decrease in cell viability was noticed. According to figure 2, it can be seen that the T47D cells showed much higher sensitivity than MCF-7 cells. Figure 2 indicates that the cell viability of HEK293 after exposure to ZnONPs showed no obvious cytotoxic effect at any concentration (p˃0.05).
In the present study, higher mortality was observed for T47D cells than MCF-7 cells and the anti-cancer effect was enhanced following the increase of ZnONPs concentration.
Real time PCR: Regulation of metastasis suppressor genes plays a prominent role in the metastasis of breast cancer. Various investigators have revealed that loss of KAI-1 and NM23 gene expression has been correlated with the degree of metastatic potentials of various types of cancers 17-21. Bozdogan et al reported that NM23 and KAI1 genes were down-regulated simultaneously in tumor progression of non-small cell lung cancer 22. Moreover, Stark et al described reduction of metastasis suppressor genes including NM23, KAI1, BRMS1, and Mkk4 in breast cancer brain metastases 23.
In the current study, T47D and MCF-7 cancer cells were treated with ZnONPs at IC50 concentration for 24 hr and quantitative real-time PCR was carried out to evaluate the mRNA levels of KAI-1 and NM23 genes. It can be seen that ZnONPs up-regulated the mRNA expression levels of these genes in T47D and MCF-7 cells. The mRNA level of KAI-1 and NM23 gene (Figure 3) was enhanced in MCF-7 cells in the treated cells compared with the untreated cells. Also, mRNA expression of KAI-1 and NM23 (Figure 4) was significantly up-regulated (3.9 and 7.6 fold, respectively) in ZnONP-exposed T47D cells as compared with the untreated control cells (p<0.001 for each). The current study suggested that ZnONPs could inhibit metastasis in T47D and MCF-7 cells through the up-regulation of mRNA level of KAI-1 and NM23 genes.
Apoptotic properties: Apoptosis is a critical physiological process of cell death determined by different morphological and biochemical hallmarks 24.
Many studies have suggested that nanoparticles can generate ROS products and activate the caspase family of proteins followed by the liberation of cytochrome c from the inter membrane space of mitochondria 25. Activation of caspase proteins, as cysteine protease, is responsible for the execution phase of apoptosis 26. Among the many proteins involved in apoptosis, caspases’ activities are critical for further investigation. The activation of caspase 8 can initiate extrinsic (Death-receptor) pathway, while the activation of caspase 9 triggers intrinsic pathway of apoptosis via mitochondria 27. To clarify the caspase activity through apoptosis, impact of ZnONPs on caspase 8 and caspase 9 activities was measured in T47D and MCF-7 cells.
The results showed that caspase 9 activity of MCF-7 and T47D cells was enhanced 2.30 and 1.70 times, respectively after treatment with ZnONPs, as compared with untreated cells, while no significant change in caspase 8 activity was observed in treated cells, comparing with those of the untreated cells (Figures 5 and 6). These results suggest that the intrinsic pathway in mitochondria via caspase-dependent pathway might be involved in nanoparticles-induced apoptosis.
In our study, significant apoptotic activity of ZnONPs was detected in comparison to necrosis in MCF-7 and T47D cells (Figure 7). The percentage of apoptotic cells following IC50 concentration of ZnONPs was 7.23 and 62.95 in MCF-7 and T47D cells, respectively. Previous reports suggest the apoptotic activity of ZnONPs in different types of cancers 28-30. The current investigation revealed that the apoptosis of cells by ZnONPs was related to the type of cancer cells. It was shown that T47D cells were less sensitive to ZnONPs than MCF-7 cells. The result of flow cytometry was in agreement with that of Hoechst 33258 staining and caspase assay and confirmed the high apoptotic potential of ZnONPs.
The cell cycle progression of cancer cells was evaluated with ZnONPs by flow cytometry. Figure 8 indicates that ZnONPs can increase sub-G1 population as compared to negative control. Interestingly, such data demonstrate that ZnONPs induce DNA fragmentation and arrest all stages of the cell cycle. Similarly, Boroumand Moghaddam et al 31 reported that the green synthesized ZnONPs induced apoptosis (sub-G1 stage) in MCF-7, with the IC25, IC50 and IC75 concentrations of nanoparticles at 24 hr incubation time.
Conclusion :
In summary, our findings confirm the previous reports about the anti-cancer properties of ZnONPs and indicate that these nanoparticles can increase the rate of MCF-7 and T47D cancer by inducing cell death. The current research confirms that ZnONPs increase the mRNA expression levels of two metastasis suppressor genes (KAI-1 and NM23). Therefore, the nanoparticles may interfere with the metastasis pathway through the up-regulation of KAI-1 and NM23. In our study, the caspase assay data of the two human breast cancer cells exposed to ZnONPs revealed significant increase of caspase 9 activities. Apoptosis assay via flow cytometry, cell cycle assay and cell morphology after treatment with nanoparticles can strongly confirm the anti-cancer properties of ZnONPs. In addition, ZnONPs can increase sub-G1 population of breast cancer cells. Overall, ZnONPs may be a potential agent for the killing of cancerous cells.
Conflict of Interest :
All of the authors report no conflicts of interests in this study.
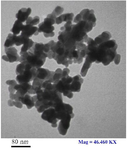
Figure 1. Transmission Electron Microscopy (TEM) image of Zn oxide nanoparticles.
|
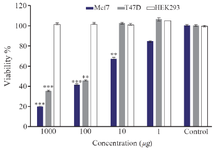
Figure 2. Cytotoxic effect of ZnONPs on cancer and normal cells at 48 hr. *p<0.05, **p<0.01,***p<0.001 indicate significant difference between nanoparticle treated cells vs. controls.
|
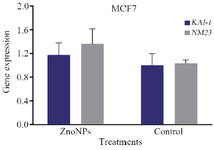
Figure 3. Impact of ZnONPs on mRNA expression levels of KAI-1 and NM23 (b) genes in MCF-7 cells.
|
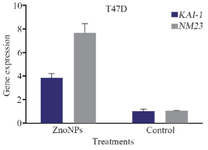
Figure 4. Impact of ZnONPs on mRNA expression levels of KAI-1 and NM23 genes in T47D cells.
|
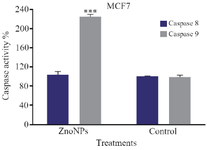
Figure 5. Effect of ZnONPs on caspase 8 and caspase 9 activities in MCF-7 cell line. * Significant differences at p<0.05.
|
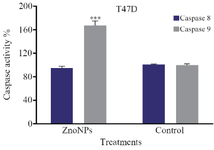
Figure 6. Effect of ZnONPs on caspase 8 and caspase 9 activities in T47D cell line. * Significant differences at p<0.05.
|

Figure 7. Flow cytometric analysis of annexin V-FITC/PI staining of T47D and MCF-7 cells treated with ZnONPs. Dot plots of annexin V/PI staining are revealed in (A) untreated MCF-7 cells, (B) untreated T47D, (C) MCF-7 cells and (D) T47D cells treated with ZnONPs
|

Figure 8. Flow cytometric analysis of the cell cycle progression of untreated MCF-7 cells (A) and treated MCF-7 (B), untreated T47D cells (C) and treated T47D cells (D) following 24 hr of treatment with ZnONPs.
|
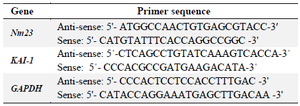
Table 1. Characteristics of the primers of KAI-1 and Nm23 and β-actin genes used in the real time PCR
|
|