Angiotensin II Differentially Induces Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 Production and Disturbs MMP/TIMP Balance
-
Yaghooti, Hamid
-
Department of Biochemistry, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
Firoozrai, Mohsen
-
School of Medicine, Iran University of Medical Sciences, Tehran, Iran, Tehran, Iran
-
Fallah, Soudabeh
-
School of Medicine, Iran University of Medical Sciences, Tehran, Iran
-
 Khorramizadeh, Mohammad Reza
Ph.D., School of Advanced Medical Technologies, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 88991118-20 Fax: +98 21 88991118-20 E-mail: khoramza@sina.tums.ac.ir
Khorramizadeh, Mohammad Reza
Ph.D., School of Advanced Medical Technologies, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 21 88991118-20 Fax: +98 21 88991118-20 E-mail: khoramza@sina.tums.ac.ir
-
Department of Medical Biotechnology, School of Advanced Medical Technologies, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Angiotensin II, the main component of the renin-angiotensin system, is associated with cardiovascular diseases such as hypertension, vascular remodeling and inflammation. Remodeling process results from dysregulation of Matrix Metalloproteinases (MMPs) and their tissue inhibitors (TIMPs). MMPs are considered as important target genes for angiotensin II. The aim of this study was to determine the effects of angiotensin II on MMP-9 and TIMP-1 production and MMP/TIMP balance in a monocytic cell type. Human monocytic U-937 cells were cultured and treated with 100 nM angiotensin II. Supernatants were analyzed for MMP-9 and TIMP-1 using ELISA and zymography methods. Real-time PCR was utilized to evaluate relative MMP-9 and TIMP-1 genes expression following treatments. Cytotoxicity potentials of treatments were determined by assaying lactate dehydrogenase leakage from the cells. Stimulation of the monocytic cells with angiotensin II significantly increased MMP-9 and TIMP-1 secretion as measured by ELISA (p<0.05). It also augmented gelatinolytic activity of MMP-9 in the conditioned media as much as 49% (p<0.05). Incubation of the cells with angiotensin II for 12 hr increased MMP-9 and TIMP-1 gene expression 2.7 and 1.8 folds, respectively (p<0.05). Angiotensin II treatments did not establish significant cytotoxic effects. In summary, our data provide further evidences that monocytic MMP-9 is a major effector of angiotensin II. It is induced more efficiently than TIMP-1 by angiotensin II that leads to MMP/TIMP imbalance. Our data also reveal the pivotal participation of these cells in pathological cardiovascular remodeling mediated by angiotensin II.
Introduction :
The Renin-Angiotensin System (RAS) and specially its primary effector, Angiotensin II (ANG), have the central role in cardiovascular and hemodynamic homeostasis. Not only RAS acts in an endocrine manner, but its
components are also present in various tissues
and vasculature to serve paracrine and autocrine functions by local ANG production. ANG can potentially affect all vascular cells including endothelial cells, Smooth Muscle Cells (SMCs), fibroblasts, monocyte/ macrophages and cardiac myocytes (1). In pathological states, ANG develops hypertension, endothelial dysfunction, inflammation and cardiovascular remodeling such as atherosclerosis and hypertrophy (2). Vascular inflammation is evident in all conditions associated with ANG-mediated vascular damage. The inflammatory responses of ANG include increase in vascular permeability, recruitment of immune cells and tissue remodeling (3).
Vascular remodeling is associated with permanent degradation and reorganization of the Extra Cellular Matrix (ECM) scaffold of the vessel wall. This process indicates the necessary participation of specialized enzymes called Matrix Metalloproteinases (MMPs). In the vessel wall, dysregulated functions of MMPs cause degradation of basement membrane, ECM and fibrous cap of plaques, that lead to impaired endothelial barrier function, infiltration of inflammatory cells, migration and proliferation of SMCs and rupture of atherosclerotic plaques (4). In physiological states, activities of MMPs are precisely regulated at the level of transcription, activation of precursor zymogen, secretion, and inhibition by endogenous inhibitors. Tissue inhibitors of metalloproteinases (TIMPs) are specific endogenous inhibitors of MMPs that participate in controlling the local activities of MMPs in tissues (5).
Extracellular matrix homeostasis is strictly tuned by a coordinated balance between the expression of MMPs and the expression of TIMPs. It has been shown that a perturbation in the balance between TIMPs and MMPs is associated with pathological turnover of extracellular matrix components in numerous cardiovascular diseases (6, 7). Major promoters of vascular remodeling, e.g. hemodynamic stimuli, oxidative stress, inflammatory and vasoactive agents regulate MMPs and TIMPs genes expression and activation; thereby alter MMP/ TIMP balance (4).
It has been demonstrated that ANG could modulate expression of MMPs and TIMPs via signaling cascades that are different in cell types (8 - 10). Monocytes are important target cells of ANG expressing both AT1 and AT2 receptors. Monocytes-macrophages have substantial roles in promoting vascular inflammation, foam cell formation and MMP secretion (11, 12).
The present study was designed to investigate the effects of ANG on the expression and secretion of MMP-9 and its inhibitor, TIMP-1, from monocytic cells. We further determined the contribution of these cells in the ANG-mediated MMP-9/TIMP-1 imbalance.
Materials and Methods :
Cell culture
Human monocytic U-937 cell line was provided from the cell bank of Pasteur Institute of Iran (NCBI). Cells were grown in RPMI-1640 containing 5% FBS, 100 IU/ml penicillin, and 100 µg/ml streptomycin in humidified atmosphere of 5% CO2: 95% air at 37 °C. Culture media were renewed three times a week. Cells were seeded in 12-well plates at a density of 1.0×106 cells/ ml in a low serum medium and were treated with either ANG (100 nM) or Lipopolysaccharide (LPS)
(100 ng/ml) (Sigma Chemical Co. St. Louis, MO). LPS, a common inducer of monocytes, was applied as a positive control in the experiments. After an overnight incubation, conditioned media were centrifuged and cell pellets and supernatants were frozen separately at -80 °C. Cell culture reagents were provided from Gibco (Invitrogen, USA).
ELISA assay
To assess the effects of ANG on MMP-9 and TIMP-1 secretion to the culture media, we measured their levels in the culture supernatants using a commercial sandwich ELISA kit (R&D systems, Minneapolis, MN). Procedures were carried out according to the manufacturer's protocol.
Zymography
This technique was applied as previously described (13). Briefly, samples of conditioned media were equally loaded onto Sodium Dodecyl Sulfate Polyacrylamide Gel (SDS-PAGE) containing 0.1% gelatin type B. Electrophoresis was carried out under non-reducing conditions. Gels were washed in 2.5% Triton X-100 twice for 30 min and incubated in substrate buffer (50 mmolar Tris-HCl, 5 mmolar CaCl2, 0.01% NaN3, pH 7.6) for 24 hr at 37 ºC. Gels were stained with 1% Coomassie blue R250 for 1 hr and was destained (45% methanol, 10% acetic acid). Places of enzymatic activity revealed as clear bands over a dark blue field. Finally, gels were photographed and analyzed by NIH ImageJ software. Data are presented as fold change relative to control.
Gene expression analysis
The expressions of MMP-9 and TIMP-1 genes in response to ANG treatment were analyzed using real-time PCR technique. RNA was isolated from the cell pellets employing FAST Pure RNA extraction kit (Takara bio inc., Japan). One µg of total RNA from each sample was used for cDNA synthesis using Primescript RT enzyme (Takara Bio Inc., Japan).
PCR amplification was performed using specific primer pairs and Taqman probes (Alpha DNA, Montreal, Canada) cited in RTPrimer data base with the following sequences: MMP-9 forward 5'-ACC TCG AAC TTT GAC AGC GAC-3', reverse 5'-GAG GAA TGA TCT AAG CCC AGC-3', probe FAM5'-TGC CCG GAC CAA GGA TAC AGT TTG TT-3'TAMRA, TIMP-1 forward 5'-ATC CGG TTC GTC TAC ACC CC-3', reverse 5'-CAG GTA GTG ATG TGC AAG AGT CC-3', probe FAM5'- AGA GTG TCT GCG GAT ACT TCC ACA GGT CC-3' TAMRA, GAPDH forward 5'-GTG AAC CAT GAG AAG TAT GAC AAC-3', reverse 5'-CAT GAG TCC TTC CAC GAT ACC-3', and probe FAM5'-CCT CAA GAT CAT CAG CAA TGC CTC CTG-3'TAMRA. Reactions were carried out utilizing Rotorgene 6000 thermocycler (Corbett research, Australia).
Shuttle PCR conditions were as follows: initial denaturation at 95 °C for 15 sec following 40 cycles of denaturation at 95 °C for 10 sec and annealing/ extension at 59, 60 and 62 °C (for TIMP-1, MMP-9 and GAPDH respectively) for 25 sec.
Target genes expressions were normalized against GAPDH expression and relative quantification analysis was performed based on ??Ct calculations. Melting curve analyses were also performed by Rotorgene 6000 software to assure specific amplifications of the favorable sequences.
Cytotoxicity assay
To determine a potential cytotoxicity effect of the ANG concentration that was used in our experiments, cell supernatants were assayed for Lactate Dehydrogenase (LDH) activity using a LDH based cytotoxicity detection kit (Roche, Germany). Procedures were performed according to the manufacturer's protocol.
Statistical analysis
The data are expressed as mean ± SEM of four independent determinations and analyzed by ANOVA followed by Tukey multiple comparison test using SPSS software version 17.
Result :
To evaluate the effects of ANG on MMP-9 and TIMP-1 secretion, we benefited from ELISA and zymography techniques. As shown in figure 1 (A and B), stimulation of the cells with ANG for 24 hr resulted in 2.4 and 1.6 folds increase in MMP-9 and TIMP-1 levels, respectively (p<0.05). For further confirmation of the results, gelatinolytic activities of conditioned media were also determined (Figure 2). Two bands were detected for each sample in zymography analysis. Cultured U-937 cells constitutively secreted MMP-2 and MMP-9 to the incubation medium in treated and control groups as revealed by gelatin zymography.
In accordance with ELISA results, ANG significantly increased the activity of MMP-9 in the medium (49%, p<0.05). LPS which was applied in the experiments as a positive control, substantially induced MMP-9 and TIMP-1 secretion.
Real-time PCR technique was used to analyze MMP-9 and TIMP-1 genes expression in response to ANG. The amplicon sizes of PCR products with the applied primer pairs were 113, 125, and 123 bp for MMP-9, TIMP-1, and GAPDH, respectively. As shown in figure 3, incubation of cells with ANG for 12 hr induced 2.7 and 1.8 folds increase in MMP-9 and TIMP-1 gene expression respectively (p<0.05). Tm values of amplicons found to be 87, 83, and 81.5 °C for MMP-9, TIMP-1, and GAPDH, respectively.
In order to calculate the cyotoxicity percentage of ANG in the applied concentration, the percentage release of LDH from the treated cells was calculated by comparing it to the maximum release of LDH achieved by lysis of the control cells using a lysis solution. The cytotoxicity percentage was measured 6.5?0.8% which was not considered significantly cytotoxic (p=0.6).
Discussion :
Alteration in MMPs and TIMPs activities and the consequent MMPs/ TIMPs imbalance, mediates pathological remodelings during atherosclerosis, myocardial infarction, left ventricular hyperplasia and heart failure as it has been shown in animal models. Ischemic cardiomyopathies, pressure and volume overload as well as neuroendocrine factors such as angiotensin II, endothelin 1, TNF-? and TGF-? can alter MMPs and TIMPs expression in a time and cell dependent manner, leading to disturbance of MMPs/ TIMPs balance in cardiovascular tissues (14).
It has been demonstrated that application of AT1 Receptor Blockers (ARBs), normalize MMPs and TIMPs expression and restores their normal balance (15). Since in in vivo studies, biopsy speciemens have been used, it could not be determined how a specific cell type contributed to the observed divergent patterns of MMP and TIMP expression. In this study we investigated the effects of ANG on the expression and secretion of MMP-9 and its tissue inhibitor, TIMP-1, in monocytes. These cells are important target cells of ANG and account for a great deal of secreted MMPs and TIMPs during cardiovascular remodeling (4, 16). We employed monocytic U-937 cells that have been used to investigate modulation of cytokines and inflammatory genes expression and corresponding signaling pathways in response to various agents (17). Yuan and colleagues used this cell to evaluate angiotensin II type 1 receptor blockade to inhibit IL-1beta production (18). This cell secretes considerable amounts of MMP-9 that can be served as a positive control of MMP-9 secretion.
In our study, the data obtained from ELISA assay, zymography analysis and gene expression experiments were in accordance in which ANG stimulation significantly augmented MMP-9 secretion. MMPs are considered as inflammatory genes that are induced through different signaling cascades in various cell types. It has been demonstrated that PKC, MAP kinases, and NF-?B signaling cascades are major pathways involving in the regulation of MMPs expression. In the promoter region of MMP-9, there are binding elements for NF-?B, AP-1, Ets-1, and STAT transcription factors (19). Earlier studies have demonstrated that ANG stimulates MMP-1, MMP-3, and MMP-9 secretion in SMCs via NF-?B and AP-1 activation in a redox sensitive manner (8). In cardiac myocytes, ANG signals mechanical stress to induce MMP-2 and MMP-14 genes expression via JNK-STAT pathway (9).
Luchtefeld and colleagues reported that ANG-induced MMP-2 secretion is NADPH oxidase-dependent in endothelial cells (10). These findings suggest that ANG can induce multiple MMPs within the atherosclerotic lesion and cardiac tissue which together can enhance neo-intima formation, plaque destabilization, hypertrophy, and heart failure. Among matrix degrading proteases, MMP-9 has a profound effect in atherosclerosis development and vascular inflammation. Furthermore genetic studies have been shown that MMP-9 gene polymorphisms are related to the presence and severity of atherosclerosis (20).
Based on our in vitro experiments, ANG challenging augmented TIMP-1 production in monocytes is revealed by ELISA and real-time PCR methods. This is in accordance with earlier studies that demonstrated the stimulatory effect of ANG in fibroblasts, aortic smooth muscle cells and heart endothelial cells through Ca2+ overload, PKC and STAT3 activation (21 - 23).
Zhang and colleagues showed that ANG could up-regulate TIMP-1 expression through activating STAT3 signaling pathway in human senescent cells. This finding indicated that ANG II-STAT3-TIMP-1 pathway might be involved in the mechanism of sclerosis in aging tissues (24). TIMP-1 was increased to compensate for the MMP-9 increase in response to ANG exposure. This concurrence could be interpreted by common binding elements in their promoters that respond in the same way toward ANG.
It should be noted that MMP-9, as an inflammatory gene, was activated more efficiently than TIMP-1 due
Acknowledgement :
This study was financially supported by the Iran University of Medical Sciences.
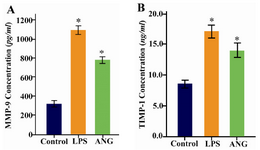
Figure 1. A) Measurement of MMP-9 B) and TIMP-1 levels in cell free conditioned media by ELISA. 1×106 serum starved cells were seeded in 12-well plates and treated either with LPS (100 ng/ml) or ANG (100 nmolar) for 24 hr. Data are expressed as mean±SEM (n=4). * p<0.05 vs. control. ANG=Angiotensin II, LPS= Lipopolysaccharide
|
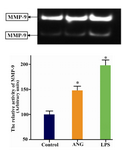
Figure 2. Zymographic determination of MMP-9 in culture media of U-937 cells. 1×106 serum starved cells were seeded in 12-well plates and treated either with LPS (100 ng/ml) or ANG (100 nmolar) for 24 hr. A representative zymogram has been shown (upper panel). Data are presented relative to control as mean±SEM (n=4) following densitometry. * p<0.05 vs. control. ANG= Angiotensin II, LPS = Lipopolysaccharide
|
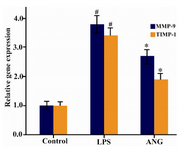
Figure 3. Analysis of MMP-9 and TIMP-1 gene expression by real-time PCR. 1.0×106 serum starved cells were seeded in 12-well plates and incubated either with LPS (100 ng/ml) or ANG (100 nmolar) for 12 hr. Gene expression data are expressed relative to control as mean±SEM (n=4). * p<0.05 vs. control, # p<0.01 vs. control. ANG=Angiotensin II, LPS = Lipopolysac-charide
|
|