Intermittent Feeding Attenuates Clinical Course of Experimental Autoimmune Encephalomyelitis in C57BL/6 Mice
-
Kafami, Laya
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Raza, Mohsin
-
Applied Neuroscience Research Center, Baqiyatallah University of Medical Sciences , Tehran, Iran
-
Razavi, Alireza
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Mirshafiey, Abbas
-
Department of Pathobiology, School of Public Health, Tehran University of Medical Sciences , Tehran, Iran
-
Movahedian, Mansooreh
-
Department of Anatomy, Faculty of Medical Sciences, Tarbiat Modares University , Tehran, Iran
-
 Khorramizadeh, Mohammad Reza
Ph.D., Tehran University of Medical Sciences, Tehran, Tel: +98 21 66462268, Fax: +98 21 66462267, E-mail: khoramza@sina.tums.ac.ir
Khorramizadeh, Mohammad Reza
Ph.D., Tehran University of Medical Sciences, Tehran, Tel: +98 21 66462268, Fax: +98 21 66462267, E-mail: khoramza@sina.tums.ac.ir
-
Department of Medical Biotechnology, School of Advanced Medical Technologies, Tehran University of Medical Sciences , Tehran, Iran
Abstract: Multiple Sclerosis (MS) is an autoimmune inflammatory, demyelinating disease of human central nervous system. Experimental Autoimmune Encephalomyelitis (EAE) is the commonly used animal model of MS. Calorie restriction has been found to reduce inflammation and autoimmune responses and promote neuroprotection. In this study we evaluated the effects of intermittent feeding protocol of the calorie restriction in a mouse model of EAE. Fifty four female mice (C57BL/6) were used in this study. The animals were divided into two dietary groups: ad libitum (AL) (n=29) with free access to food and water and intermittent feeding (IF) (n=25) with access to food on alternate days. After 8 weeks, EAE was induced in animals by immunization with MOG antigen (Hooke labs, Lawrence, MA, USA) subcutaneously. AL and IF groups were then further divided into two groups each: AA (ad libitum until the end of study) (n=16) and AI (subjected to intermittent feeding regimen after immunization day) (n=13). The IF group was divided into II (continued intermittent feeding regimen until the end of study) (n=13) and IA (changed to AL regimen after immunization day) (n=12). All the animals were behaviorally monitored for 35 days after immunization and observed daily for the signs and severity of disease with EAE scoring scale [0-5] and cumulative disease index (CDI) score. Intermittent feeding significantly reduced the incidence of EAE in IF groups (AI 0%, II 18.5%, IA 22.2%, p<0.05). In addition, intermittent feeding significantly delayed the onset of EAE in AI group (p<0.05) and also, intermittent feeding significantly reduced the severity of disease in II and IA groups (AA vs. II, p<0.05 & AA vs. IA p<0.05) groups. The CDI was also significantly reduced in intermittent feeding fed groups [AI, II and IA compared to AA group (P<0.05, <0.01, <0.05 respectively)]. Intermittent feeding regimen protocol of the calorie restriction significantly suppressed EAE incidence, induction, and severity. The results of this study suggest possible role of intermittent feeding in the treatment of Multiple Sclerosis patients.
Introduction :
Multiple sclerosis (MS) is a chronic autoimmune demyelinating disease of the central nervous system (CNS) in human. However, while the disease has been known
for over one hundred years, its exact etiology still remains unresolved. Currently available scientific literature suggests that MS is the result of immune system activation by various agents in genetically susceptible individuals that initiates a pathogenic cascade eventually leading to the progressive destruction of the myelin sheath and axonal damage (1). Therapies for the treatment of MS are not curative and only effective in decreasing the relapse rates and even less effective in preventing the disease progression. One of the novel therapies for MS can be targeting the underlying mechanisms of chronic disability (2). Experimental Autoimmune encephalomyelitis (EAE) is the most commonly used animal models for studying MS. To date, EAE has been invaluable model for investigating the pathogenesis of MS and developing new therapies for MS treatment.
Calorie restriction is limiting dietary energy intake without a reduction in essential nutrients or causing malnutrition (3). It improves health and retards aging (4). Calorie restriction stabilizes immune system to the optimal level (5). Several studies have shown that calorie restriction suppresses autoimmune processes in human and experimental models of autoimmune diseases including lupus erythematosus, arthritis rheumatoid, Sjogren's syndrome (6 - 10), and experimental autoimmune encephalomyelitis (11 - 13). Calorie restriction promotes neuroprotection and induces neurogenesis and protects neurons against various excitotoxic insults (14). It also improves cognitive functions of brain, reduces age-associated deficits of brain function and significantly reduces vulnerability to injury and disease (15). It was demonstrated that calorie restriction specifically reduces incidence of diseases including Alzheimer disease, Parkinson’s disease, Huntington disease, stroke in animals and humans (16 - 18).
Two different paradigms have been employed in studies on calorie restriction: feeding animals with between 25-65% of food ad libitum every day or feeding them ad libitum every other day entitled "intermittent feeding". Intermittent feeding has been used for studying the effects of calorie restriction on several diseases (19). It has been demonstrated that intermittent feeding is more effective than the other paradigm in suppressing immunological and neurological diseases (19).
In this study, we evaluated the effects of intermittent feeding on clinical course and severity of EAE using C57BL/6 mice.
Materials and Methods :
Animals:All the experiments were carried out on 4-5 weeks old female C57BL/6 mice (n=54). The animals were purchased from Tehran Pasteur Institute. They were housed in groups of five and all were maintained under constant conditions of temperature (22—24 ?C) and 12 hr light (7.00-19.00 hr/ 12 hr dark cycle. Animals were kept for about 1 week for acclimation time. All the animal treatments and experiments were carried out according to the policies of the Society for Neuroscience (20). The research project was approved by the Medical Research Ethics Committee of the Tehran University of Medical Sciences.
Experimental groups and EAE induction:Animals (n=54) were age-matched and divided into two main groups as follows: AL (n=29) that received food ad libitum and IF (n=25) that were subjected to intermittent feeding before immunization. Both groups were fed with a standard laboratory mouse chow and were weighed twice a week before disease induction. All animals received water ad libitum. At week 8, AL group was divided into two groups as follows: AA group (n=16) that continued on ad libitum diet until the end of experiment period and AI group (n=13) whose diet was changed to intermittent feeding from this time until the end.
Mice in IF group, was also divided into II (n=13) and IA (n=12) groups that their diet continued as before until the end of the study or changed to ad libitum from this time to the end, respectively. Thirty eight animals (AA=12, AI=9, II=9, IA=8) were immunized with Hooke kits (Hooke labs, EK-0115, Lawrence, MA, USA) according to manufacturer’s instructions. Briefly, after deep anesthesia with ether, 0.1 ml MOG-CFA emulsion was injected to the flanks of each mouse (0.2 ml/ animal) subcutaneously. Immediately and 24 hrs later each mouse received pertussis toxin intraperitoneally (0.1 ml/animal, Ip). Rest of the animals (n=16; 4 animals/ group) was used as control that received saline.
Clinical evaluation:One day before immunization and from 7th day to 35th day post-immunization, the animals were daily evaluated for the signs of EAE using the 10 score system as follows: 0, no clinical disease; 0.5, partial tail paralysis; 1.0, complete tail paralysis; 1.5, complete tail paralysis and discrete hind limb weakness; 2.0, complete tail paralysis and strong hind limb weakness; 2.5, unilateral hind limb paralysis; 3, complete hind limb paralysis; 3.5, hind limb paralysis and forelimb weakness; 4.0, complete paralysis (tetraplegia); 5.0: moribund or dead (21, 22). Three clinical parameters were analyzed to compare the course of EAE: Severity of disease as Cumulative Disease Index (CDI) score was calculated as the average of the sum of the daily clinical scores for each mouse. Disease Onset: averaging the first day of clinical signs for each mouse in the group. Peak disease score: averaging the highest individual score for each mouse in the group (22).
Statistical analysis:Statistical significance between experimental groups was calculated as follows: Daily clinical scores of AI, II and IA groups were compared with AA group and analyzed with Mann-Whitney test. CDIs, days of onset and peak disease score were compared among the groups and analyzed by one way ANOVA test. Values of p<0.05 were considered to be statistically significant.
Result :
Intermittent feeding regimen completely prevented the EAE in AI group that received intermittent feeding from the day of immunization with incidence of 0. AA animals developed the disease with the incidence of 75% and CDI of 1.05±0.69. II and IA groups that received intermittent feeding developed the signs of the disease with the incidence of 22%, 50% and CDI of 0.22±0.45 and 0.44±0.69, respectively. The overall clinical course of the disease during the experiment was significantly lower in intermittent feeding treated groups including AI, II groups compared with AA group with p<0.05 and p<0.01, respectively. Overall intermittent feeding treated groups compared with AA group showed the disease with lower incidence and severity (p< 0.01).
Daily clinical signs of the disease were recorded by a blind person using 10-point scoring system for 35 days postimmunization (Figure 1). None of mice in AI group developed the disease during the study (Figure 1A). Intermittent feeding inhibited the disease course when started immediately after immunization. Compared to AA group, II and IA groups developed the disease with less severity (Figures 1B and C, respectively).
To test whether intermittent feeding can postpone the disease initiation; day of onset for each group was calculated by averaging the first day of clinical signs of animals in each group (Figure 2). AA animals developed the disease after 15.44±4.36 days. Whereas, II and IA groups showed the signs of the disease on 18.50±0.70 days and 22.25±11.26 days, respectively. The differences between intermittent feeding and ad libitum treated groups are not significant (p>0.05).
To examine the effects of intermittent feeding on peak of disease, the score of disease peak for each group was calculated with averaging the highest score recorded for animals in each group (Figure 3). AA animals developed the disease with 2.53±1.32 score at disease peak. II and IA groups showed disease peak with 0.5±0.94 and 1.12±1.16 score, respectively. The differences between intermittent feeding treated groups and AA group at disease peak are not significant (p>0.05).
Discussion :
The results of this study indicate that intermittent feeding has protective effects in mouse model of EAE. Overall, our results on clinical evaluation of EAE indicate that compared with AA group, animals subjected to intermittent feeding got less severe disease.
The beneficial effect of intermittent feeding seen in EAE model is mainly mediated by anti-inflammatory, immunomodulatory and neuroprotective effects of calorie restriction. Calorie restriction maintains the cellular components of immunity to normal and keeps mediators and antibodies to the level related to young and healthy conditions (7). Several investigators have reported that calorie restriction can prevent autoimmune diseases in animals and human beings (5, 6, 8 -10).
Our results suggest that intermittent feeding might postpone the disease onset and decreases the course of the disease at peak of disease. But there were no significant differences between AA group and intermittent feeding groups.
Esquifino et al reported that calorie restriction reduced the clinical course of EAE in Lewis rats when the animals were kept on moderate and severe calorie restriction, 33 and 66%, respectively and demonstrated inhibition of the disease in second group subjected to severe calorie restriction (11, 12). Another study using two different mouse models of EAE, the SJL and the C57BL/6 mice, also reported the suppression of EAE with calorie restriction (13).
Our results indicate that intermittent feeding can inhibit the disease incidence when started immediately after immunization, whereas chronic intermittent feeding can prolong the course of the disease suggesting protective effect of calorie restriction in EAE. In our study, animals in AI group did not show any signs of the disease.
One mechanism by which intermittent feeding can acutely suppress the disease is by increasing the endogenous production of glucocorticoids (13). Glucocorticoids are the strong inhibitors of immune system. The acute stress induced in animals by putting them on diet immediately after the immunization results in secretion of glucocorticoids that in addition to the metabolic effect of intermittent feeding on increasing the levels of glucocorticoids can suppress the disease (23).
Further studies are suggested on the supersession of EAE induction with intermittent feeding administered in parallel with immunization. Levels of glucocorticoids and other inflammatory mediators such as IL-6, TNF a at the time of immunization in this protocol might be useful to confirm the results observed in this study.
The results of this study demonstrate a novel approach for application of calorie restriction in EAE and suggest further studies which include additional control groups and investigation of cellular and molecular mechanisms.
Acknowledgement :
This study was supported by the research grant number of 4888 from the Deputy of Research, Tehran University of Medical Sciences.

Figure 1. Effects of ad libitum and intermittent feeding on clinical course of the disease in various experimental groups
Daily clinical scores of AI, II and IA groups were compared with AA group using Mann-Whitney test. Values shown are means ± SD. A; IF diet ameliorated the disease when administered at the day of immunization. Open circles and black squares represent the mean clinical score for AA and AI groups, respectively. B, C; intermittent feeding decreased the severity of the disease in II and IA groups comparing with AA group. Open circles, black circles and black triangles represent AA, II and IA groups, respectively (p<0.05, p<0.01)
|
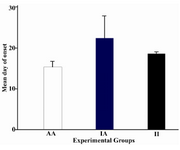
Figure 2. Effects of intermittent feeding on day of disease onset in various groups
Days of onset were compared among the experimental groups and analyzed by one way ANOVA test. Values shown are means ±SD. White column, Plain column and black column represent mean day of onset for AA group, IA group and II group, respectively. AI group was not indicated in this figure because of no incidence. There is no significant difference between IF and AL groups (p>0.05)
|
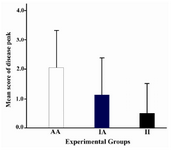
Figure 3. Effects of intermittent feeding on peak of disease in various experimental groups
Score at peak of disease were compared among the experimental groups and analyzed by one way ANOVA test. Values shown are means ±SD. White column, Plain column and black column represent peak disease score for AA group, IA group and II group, respectively. AI group was not indicated in this figure because of no incidence. There is no significant difference between IF and AL groups (p>0.05)
|
|