Development in Immunoprophylaxis against Rabies for Animals and Humans
-
 Nandi, Sukdeb
Ph.D., Virology Laboratory, Center for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute (IVRI), Izatnagar, U.P., India, E-mail:snandi1901@yahoo.com
Nandi, Sukdeb
Ph.D., Virology Laboratory, Center for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute (IVRI), Izatnagar, U.P., India, E-mail:snandi1901@yahoo.com
-
Virology Laboratory, Center for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute (IVRI) , Izatnagar, U.P, India
-
Kumar, Manoj
-
Virology Laboratory, Center for Animal Disease Research and Diagnosis (CADRAD), Indian Veterinary Research Institute (IVRI) , Izatnagar, U.P, India
Abstract: Rabies is a fatal neurological disease and a persistent global problem. It is spread primarily by domestic dogs but other canid, viverrid (skunks and raccoons) and chiropteran species are considered as the most efficient vectors of the disease. Since dogs are the main perpetuator of rabies, special attention has to be given to bring all the dogs including unauthorized stray dogs under immunization umbrella in order to control rabies. Vaccination is the only way to combat the disease before and after exposure or infection as there is no treatment available once the symptoms have appeared. After the first crude nerve tissue vaccine developed by Pasteur in 1885, a number of rabies vaccines for animal and human use have been developed with varying degree of safety and efficacy over the years. Presently, cell culture based inactivated rabies vaccines are largely used in most of the parts of the world. However, these vaccines are too expensive and unaffordable for vaccination of people and animals in developing countries. The comparatively cheaper inactivated nerve tissues vaccines can cause serious side-effects such as autoimmune encephalomyelitis in inoculated animals and production has been discontinued in several countries. Although attenuated live vaccines can efficiently elicit a protective immune response with a smaller amount of virus, they sometimes can cause rabies in the inoculated animals by its residual virulence or pathogenic mutation during viral propagation in the body. New-generation rabies vaccines generated by gene manipulation although in experimental stage may be a suitable alternative to overcome the disadvantages of the live attenuated vaccines. So, awareness must be created in general public about the disease and the cell culture based vaccines available in the market should be recommended for wide scale use to prevent and control this emerging and reemerging infectious disease in foreseeable future.
Introduction :
Rabies, an acute fatal encephalomyelitis remains as one of the most feared and dreadful zoonotic disease in the world. It is the most important viral zoonosis recognized today because of its global distribution, incidence, veterinary and human health costs and extremely high case fatality rate. All the mammals right from a small mouse to a massive elephant are infected with the disease. Rabies is enzootic in both wild and domestic animals and poses a potential threat to human beings. In South East Asian Region (SEAR) member countries, rabies is a serious problem and account for approximately 80% human deaths in the world (1 - 3). The incidence of rabies is particularly high in Bangladesh, Pakistan and India followed by moderate incidence in Nepal and Myanmar and mild in Bhutan, Thailand and Indonesia (4 - 6).
Rabies is endemic in the countries where more than 2.5 thousand million people live. It is estimated that each year at least 55,000 people die from rabies and more than 10- 12 million people receive post exposure vaccination against this disease (7). Children aged 5- 15 years are at particular risk. More than 99% of all human deaths from rabies occur in Africa, Asia and South America (8). India alone reports 30,000 deaths annually. The disease is commonly transmitted by the bite of rabid animals usually carnivorous animals. In human beings 90% cases occur due to the bite of rabid dogs and 10% are due to the bite of other animals, aerosol transmission and transplantation of cornea and other organs.
Dogs are presumed to be the main transmitter in India due to high density of dog population. It is estimated that the dog population is around 25 million in India and 3/4th of all human rabies cases occur in villages and the incidence is about five times more in males compared to females (9, 10). The other domestic animals like cat, cattle, horse, sheep, goat etc may be victims of rabies and transmit it to man (6). In India, Andaman and Nicobar Islands and Lakshadeep are rabies free. The distribution of rabies cases is not uniform and states like Nagaland, Manipur and Sikkim have very low incidence of hydrophobia due to wide dog and human ratio (11 - 13). In India, rabies infections also prevail in wild animals like wolf, fox, mongoose, jackal, hyenas etc (9).
The frugivorous, insectivorous and vampire bats can feed on blood of man and animals and transmit the disease in parts of Latin America (Brazil, Mexico, Venezuela, Trinidad, Tobago) and U.S.A.
The annual cost of rabies has been estimated to be US $ 583.5 million and livestock losses is in the tune of US $ 12.3 millions in Asia and Africa. Dog rabies is present in 87 countries and accounts for major cause of all human rabies cases. However, many countries like Japan, U.K, Denmark, Sweden, Greece, Ireland, Iceland, Portugal, New Zealand, Australia, Switzerland, Finland, Norway, France, Belgium, etc are rabies free (14, 15).
Rabies has the dubious distinction of having the highest case fatality rate of all known infectious diseases. Rabies can be prevented by administration of potent and efficacious rabies vaccines both in pre and post exposure cases (16). It is evident that pre and post exposure use of cell culture rabies vaccines has dramatically reduced the incidence in certain countries (7). In Thailand, administration of Post Exposure Prophylaxis (PEP) has reduced the human rabies cases by 80% in 15 years (17). Other developing countries such as India, Sri Lanka and Philippines have adopted and promoted the use of economical low dose intradermal antirabies vaccination regimen using cell culture rabies vaccine (18). Currently, most of the pet dogs and cats are vaccinated against rabies but rabies infection may occur due to the vaccine failures, immuno-compromised animals, and presence of intercurrent diseases and sometimes from the asymptomatic carriers due to the close association between pets and owners (9).
Although a number of countries in the world<
Discussion :
Rabies, a dreadful and terrifying disease causing neurological symptoms with high mortality is not only a national but also a global problem. Significant advances have been made by the multi-disciplinary sectors of immunology, vaccinology, molecular virology and epidemiology thus allowing a far greater understanding of rabies virus circulation. Rabies is a disease for which all the necessary remedies exist unlike the situation with many other diseases like dengue, chikungunya etc. It is possible to prevent, control and treat rabies with the safe and effective cell culture produced rabies vaccines or anti-rabies globulins. In spite of this, WHO records more than 55,000 human deaths from rabies each year mostly due to infection by classical rabies virus (genotype 1, serotype 1) from dogs. The number of cases in humans and animals is still believed to be underestimated due to poor or under reporting in many countries in the world. Continuing molecular epidemiological and surveillance studies are necessary to trace the spillover transmission from reservoir species to non-reservoir animals and humans and also to monitor the emergence of specific rabies strains into new species and geographical area, which to a large extent is often prompted by human activities viz., movement of wildlife and importation of animals. Bat rabies epidemiology should be more comprehensively explored so that precise risks to the health of humans and domestic carnivores can be identified and effective and efficient disease prevention measures can be applied to those who handle the bats. A further more ambitious goal is to co-ordinate all efforts from all sectors to increase rabies surveillance programmes particularly in Africa and Asia and thus ultimately to decrease the incidence of rabies in the continents.
Ideally there should be a national reference laboratory for rabies diagnosis with its branches in every state of the country. The destruction of all stray and feral dogs, which is usually very unpopular with the general public and strong opposition from animal activists and animal ethics committee, does not in the long term constitute a realistic method of disease control. This method has been failed in some countries in the world. Pet dogs usually receive rabies vaccinations when they are 3 months old, but very often they become infected before they reach to this age. The stray and unauthorized dogs which are often not brought under the immunization campaign harbour the rabies virus. They act as carriers and transmit the rabies virus to other susceptible animals and humans. It has been reported that vaccination before the age of 3 months is effective, even in an animal with maternal antibodies. Puppies should be vaccinated along with adult dogs during any mass parenteral vaccination campaign. This would help to broaden vaccine coverage and reduce the incidence of rabies in children.
In India, the rabies in animals and humans is a real problem and lot of efforts have been attempted to control the disease. A large number of cases are still reported with a certain percentage of casualties. The currently available cell culture rabies vaccines have been proved to be safe, immunogenic, potent and efficacious when produced and used according to WHO recommendations. The development and proper use of vaccines has undoubtedly enabled millions of human lives to be saved from a dreadful disease. However, the high cost of the vaccines, lack of awareness of public about the usefulness of administering these vaccines for pre and post exposure cases and limited availability in many regions of the world are all factors that prevented the cell culture rabies vaccines to be utilized in full potential to benefit humankind. However, with the advancement of times and production of vaccines by many manufacturing companies and the use of I/D administration of rabies vaccines, the cost of the vaccines would be reasonable and within the reach to the common people and preve
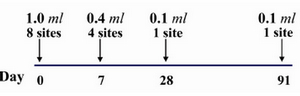
Figure 1. Details of the eight site intradermal rabies post exposure vaccination regimen (PCECV and HDCS)
|
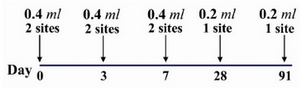
Figure 2. Details of the two site intradermal rabies post exposure vaccination regimen (PCECV and HDCV)
|
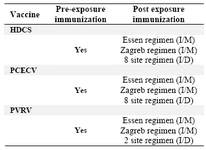
Table 1. WHO recommendation on immunization of humans against rabies
|
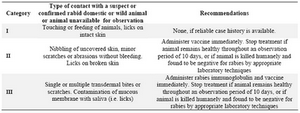
Table 2. WHO guidelines for post-exposure vaccination/treatment
|
|