The Cytotoxicity of Dacarbazine Potentiated by Sea Cucumber Saponin in Resistant B16F10 Melanoma Cells through Apoptosis Induction
-
 Baharara, Javad
Research Center for Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran, TelFax: +98 5138437092, E-mail: baharara@yahoo.com
Baharara, Javad
Research Center for Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran, TelFax: +98 5138437092, E-mail: baharara@yahoo.com
-
Department of Biology, Research Center for Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran
-
Amini, Elaheh
-
Department of Animal Biology, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran
-
Nikdel, Najme
-
Research Center for Applied Biology, Mashhad Branch, Islamic Azad University, Mashhad, Iran
Abstract: Background: Malignant melanoma is a highly aggressive malignant melanocytic neoplasm which resists against the most conventional therapies. Sea cucumber as one of marine organisms contains bioactive compounds such as polysaccharide, terpenoid and other metabolites which have anti-cancer, anti-tumor, anti-inflammatory and antioxidant properties. The present study was designed to investigate the anticancer potential of saponin extracted from sea cucumber Holothuria leucospilata alone and in combination with dacarbazine on B16F10 melanoma cell line.
Methods: The B16F10 cell line was treated with different concentrations of saponin (0, 4, 8, 12, 16, 20 µg/ml), dacarbazine (0, 1200, 1400, 1600, 1800, 2000 µg/ml) and co-administration of saponin-dacarbazine (1200 da+8 sp, 1200 da+4 sp) for 24 and 48 hr and the cytotoxic effect was examined by MTT, DAPI, acridine orange/propodium iodide, flow cytometry and caspase colorimetric assay.
Results: The results exhibited that sea cucumber saponin, dacarbazine, and co-administration of saponin-dacarbazine inhibited the proliferation of melanoma cells in a dose and time dependent manner with IC50 values of 10, 1400 and 4+1200 µg/ml, respectively. Morphological observation of DAPI and acridine orange/propodium iodide staining documented typical characteristics of apoptotic cell death. Flow cytometry assay indicated accumulation of IC50 treated cells in sub-G1 peak. Additionally, saponin extracted induced intrinsic apoptosis via up-regulation of caspase-3 and caspase-9.
Conclusion: These results revealed that the saponin extracted from sea cucumber as a natural anti-cancer compound may be a new treatment modality for metastatic melanoma and the application of sea cucumber saponin in combination with dacarbazine demonstrated the strongest anti-cancer activity as compared with the drug alone.
Introduction :
Melanoma is the second most common cancer in the world which has been considered as a serious public health problem 1. This disorder is the most aggressive form of all skin cancers which have influenced the majority of human beings per year 2. Sun exposure, particularly UVB and UVA, was proposed as a potential inductive agent of melanoma malignancy 3. Nevertheless, although UV radiation is the leading cause of skin cancer, other causative agents consisting of viruses, mutagens, chemicals and genetic abnormality were considered important in complexity of this disorder 4.
Currently, due to high potential of metastasis in melanoma and low efficacy of conventional therapeutic methods, development of alternative methodologies with enhanced safety and efficacy to overcome this challenge is urgently needed 5. Drug resistance in melanoma mainly occurred through dysregulation of apoptosis, whereas drug transport, detoxification, and enhanced DNA repair may also exert a function 6. Therefore, the apoptosis induction deliberated the major target of anti-cancer chemotherapeutic strategies 7. Vincristine and vinblastine were proposed as popular natural based anti-tumor drugs which play an important role in cancer chemotherapy 8. Dacarbazine, temozolomide and cisplatin are used as common chemotherapeutic agents for melanoma treatment; although these modalities produce low response rates approximately less than 25% 9.
Dacarbazine is the first-line anti-cancer drug against melanoma which reveals anti-cancer effect via methylation of nucleic acids or direct DNA damage, however, resistance to conventional chemotherapy is considered the main obstacle to the treatment of this malignancy 10.
The studies exhibited that one of the approaches to alter the pharmacokinetic profile and toxicity related to this drug is combination therapy; however, it is well documented that naturally occurring compounds can be promising in cancer prevention due to low cost and minimum side effects. Hence, it is necessary to identify whether novel combination therapies are superior to single-agent dacarbazine 11.
High interest for discovering useful therapeutics from marine organisms has created novel opportunity to overcome cancer remedy challenge. It is reported that novel metabolites with potent pharmacological properties have been discovered from marine origin to fight cancer 12. Saponins are naturally occurring surface-active glycosides which are divided in two main groups of triterpenoid and steroids. These secondary metabolites are mainly derived from herbal extracts but also from some marine organisms which have been used widely in drug discovery due to their bio medicinal characteristics 13.
The sea cucumbers are known as valuable sources of biologically active substances, which have gained interest among folk medicine researchers due to their use in the treatment of chronic inflammatory diseases 14. Therapeutic properties and potential health benefits of sea cucumbers can be attributed to the presence of an extensive array of bioactive compounds including chondroitin sulfates, sulfated polysaccharides, sterols, cerebrosides, peptides, triterpenoid glycosides (saponins), etc. 15.
These compounds are appealing because of their natural origin, long history of use as food, and lack of toxic effects. Recent studies have reported that sea cucumber saponin from various species can cause growth arrest against several types of cancerous cells including liver, lung, cervix, ovarian and melanoma 16.
The anti-neoplastic efficacy of natural saponins to trigger the apoptotic paradigm and scavenge free radicals constitutes a leading area of scientific oncological research; hence, cancer methodologies require the development of natural antitumor saponin compounds with minimum impact from side effects 12,17.
In the previous study, it was shown that sea cucumber saponin extracted from Holothuriz leucospilota (H. leucospilota) can exert toxicity on MCF-7 cancer cells and result in over expression of Bax and down regulation of Bcl-2 (as anti-apoptotic and as one of main pro-apoptotic factor of Bcl-2 family), leading to apoptosis administration in MCF-7 breast cancer cells 18.
Since scientific information on anti-melanoma effect of combination chemotherapeutics (Dacarbazine) and natural based bioactive compounds (extracted sea cucumber saponin) is still rather scarce, with this objective in mind, the present study was directed toward combination treatment of dacarbazine with sea cucumber saponin extracted from H. leucospilota to potentiate the cytotoxic effect of the drug in B16F10 melanoma cells.
Materials and Methods :
Chemicals: B16F10 melanoma cell line was purchased from NCBI (National Cell Bank of Iran). RPMI-1640 medium, FBS (Fetal Bovine Serum), trypsin-EDTA and antibiotic (Penicillin-streptomycin) were purchased from Gibco (USA). MTT (3-[4, 5- dimethyl thiazol-2-yl]-2, 5-diphenyl tetrazolium bromide) and DAPI were obtained from Applichem (USA). Propodium Iodide (PI) and Acridine Orange (AO) were obtained from Sigma (USA). Caspase-3 and caspase-9 colorimetric assay kits were purchased from Abcam (England).
Extraction and identification of crude saponin: Crude saponin was isolated from the body wall of sea cucumber, Holothuria leucospilota according to the method performed by Hu et al 19. Briefly, the ground sea cucumbers were extracted using methanol, dichloromethane and n-butanol, sequentially. The concentrated n-butanol fraction was loaded on diaion HP-20 and rinsed with dionized water, 80 and 100% ethanol. The 80% ethanol fraction was collected by evaporation as crude saponin and saponin identification was performed using TLC, erythrocyte lysis and FTIR which was reported previously 18.
Cell culture and MTT assay: For evaluation of cytotoxic effect of the sea cucumber saponin against melanoma cells, B16F10 cells were cultured in 96-well plate and treated with different concentrations of sea cucumber extracted saponin (0, 4, 8, 12, 16 μg/ml), dacarbazine (0, 1000, 1200, 1400, 1600, 1800, 2000 μg/ml) and synergistic treatment of saponin sea cucumber and dacarbazine (0, 1200 da+8 sp, 1200 da+4 sp μg/ml) for 24 and 48 hr. Then, cell viability was measured using 3-(4, 5-dimethylthiazol-2-yl)-2 and 5-diphenyltetrazolium bromide (MTT) assay. In this method, MTT and DMSO were added according to the company’s protocol, respectively. The absorbance of each well was measured at a wavelength of 560 nm by a spectrophotometer (Epoch, USA).
Cell morphological assessment: In order to investigate the effect of saponin sea cucumber, dacarbazine and synergistic treatment of saponin and dacarbazine in cell morphology, B16F10 cells were seeded in 24 well culture plates (105 cells/well) in RPMI supplemented with 10% FBS, after 24 hr adherence of cells. Treatment was performed at a different concentration. After 48 hr, the cells were examined under inverted microscope (Olympus, Japan).
DAPI staining: 4’, 6-diamidino-2-phenylindole dihydrochloride staining was used to examine the nuclear fragmentation. In this assay, the B16F10 cells were incubated with or without IC50 concentrations of sea cucumber saponin, dacarbazine and synergism treatment for 48 hr. Then, the cells were stained with DAPI and incubated for 10 min and the morphology of nuclei was observed under fluorescence microscopy (Olympus, Japan).
AO/PI staining: The morphological changes of apoptosis were evaluated by fluorescence microscopy. Briefly, the cultured cells were treated with IC50 concentration of saponin, dacarbazine and combination treatment for 48 hr. Then, the cells were rinsed with PBS. About 10 μl of the cell pellets were stained with a 10 μl fluorescent dye mixture containing equal volumes (100 μg/ml) of AO and PI. Finally, the cells were observed under a fluorescence microscope (Olympus, Japan).
Detection of apoptosis: To verify apoptosis in B16F10 treated cells, PI assay was performed. Exponentially growing cells were seeded and exposed with IC50 concentrations of saponin, dacarbazine and both of them for 48 hr. Then, they were centrifuged and mixed with 700 µl PI solution for 30 min in the darkness. Next, flow cytometric analysis was conducted using a FACScan laser flow cytometer (FACS Calibur, Becton Dickinson, USA).
Caspase assay: The enzymatic activities of caspase-3 and caspase-9 were measured using colorimetric caspase assay. The cells (1×106) were seeded in 6-well plate and exposed with inhibitory concentrations of saponin, dacarbazine and combination treatment. Treated cells were centrifuged and incubated in 50 µl lysis buffer for 10 min on ice. After centrifugation, the supernatants were incubated with 20 µl caspase-3 and caspase-9 substrate at 37oC for 2 hr. Absorbance of the chromophore pNA separated from the caspase substrates was detected using Epoch spectrophotometer at 405 nm.
Statistical analysis: The statistical analysis was performed by SPSS, ANOVA software and p<0.05 was considered significant.
Results :
Cytotoxic assay: To evaluate cytotoxicity of saponin, dacarbazine and synergistic treatment of saponin and dacarbazine on viability of B16F10 melanoma cells, MTT assay was examined. The results showed that saponin, dacarbazine and synergistic treatment had a marked dose-dependent effect on viability of B16F10 cells. The IC50 concentrations of saponin and dacarbazine were determined 10 and 1400 µg/ml (p<0.01), respectively. However, for a simultaneous treatment with both saponin and dacarbazine, low toxic concentration of 4 μg/ml saponin plus 1200 µg/ml dacarbazine for 48 hr caused an approximately 50% B16F10 cell death. Meanwhile the combination treatment of 8 µg/ml of saponin and 1200 µg/ml of dacarbazine and 10 µg/ml of saponin and 1000 µg/ml of dacarbazine reduced cell viability to 36.4, 28.7% in B16F10 cancer cells in 48 hr treatment (p<0.001). Consequently, treatment with sea cucumber saponin increased cytotoxicity of dacarbazine and caused more cytotoxicity in murine melanoma cells (Figure 1).
Monitoring of cell morphology: The morphology of untreated and treated melanoma cells has been shown in figure 2A. The apoptosis morphological changes include cell rounding, reducing cell volume, cytoplasmic blebbing, plasma membrane distortion as well as nuclear condensation and apoptotic bodies. The morphological observation indicated that IC50 concentration of saponin, dacarbazine and mixture of saponin and drug conducted apoptosis changes in morphology of melanoma cells including cell shrinkage, reducing cell volume and irregularities in cell contour and size which are all well known as the apoptotic characteristics (Figure 2A).
Detection of apoptosis by DAPI staining: DAPI staining as fluorescence DNA binding agent was used for evaluating nuclear apoptosis detection. Based on morphological observation from DAPI staining, DNA fragmentation occurred in IC50 concentration of saponin as well as IC50 concentration of dacarbazine and combination treatment (Figure 2B).
Monitoring morphological characterization of apoptosis by AO/PI staining: Apoptosis is the crucial cell death mechanism induced in response to cytotoxicity of chemotherapeutic drugs in cancer cells. AO/PI method is used to monitor apoptotic, necrotic and alive cells by their color (green, yellow, orang and red for alive, early apoptotic, late apoptotic and necrotic cells), respectively. When the treated cells were stained with AO/PI, apoptotic death was observed in cells incubated with IC50 concentration of saponin, dacarbazine and combination treatment (Figure 2C).
Flow cytometry analysis: In apoptotic cells, appearance of a sub-G1 peak is believed to be the result of endonuclease activation which is not observed in necrotic cells. Figure 3 shows that IC50 concentration of saponin, dacarbazine and synergistic treatment increase the sub-G1 peak (apoptotic peak). These results supported this idea that apoptotic cell death is involved in cytotoxicity of saponin, dacarbazine and combination treatment in B16F10 cells (Figure 3).
Activation of caspase-9 and caspase-3 in B16F10 cells: The caspase-cascade has an essential role in apoptosis which is divided as apoptosis activator (caspase-9) and apoptosis executioner (caspase-3). As exhibited in figure 4, caspse-3 and caspase-9 activation was significantly enhanced in a dose dependent manner with IC50 value of saponin, dacarbazine and synergistic treatment compared with the untreated cancer cells. Moreover, in comparison to treatment groups, the caspase activity was markedly increased in combination treatment groups treated with 4 μg/ml saponin, 1200 µg/ml dacarbazine and 8 μg/ml saponin/ 1200 µg/ml dacarbazine compared to 1400 μg/ml dacarbazine alone indicating that synergistic treatment with sea cucumber saponin and dacarbazine induced more potent caspase activation in B16F10 cells as compared with drug treatment. Thus, these results elucidated the involvement of intrinsic pathway in cytotoxicity of saponin, dacarbazine and combination treatment in melanoma cells.
Discussion :
Malignant melanoma arises from unregulated cell growth which is the leading cause of morbidity in the world. In spite of multiple conventional chemotherapeutic strategies for melanoma treatment, this condition remains as one of main oncological research fields 20. Dacarbazine is a standard antineoplastic drug commonly used for treating melanoma, ,though this drug may induce side effects, because it restricts normal cell proliferation 5.
In the present study, it was aimed to evaluate the cytotoxicity of sea cucumber saponin, focusing on reduction of the cytotoxic effect of dacarbazine on melanoma cancer cells. As shown in this study, treatment of B16F10 cells with sea cucumber saponin and dacarbazine presented a dose-dependent inhibitory effect on melanoma cell viability. In addition, the findings from fluorescence microscopy and flow cytometry confirmed that the cytotoxicity of sea cucumber saponin and dacarbazine alone and in combination was related to apoptosis and dacarbazine along with sea cucumber saponin elicited intrinsic apoptosis pathway which was verified through caspase-3 and -9 activation.
Chemotherapeutic modality using cisplatin, dacarbazine, temozolomide, doxorubicin and 5-FU which are used extensively as chemotherapeutic agents in numerous cancer therapies, have not completely prevented recurrence and metastasis of tumors 21.
One of the main problems with dacarbazine is associated with its cytotoxicity in normal cell growth. Meanwhile, dacarbazine renders the activation of the extracellular signal-regulated kinase pathway and up-regulation of IL-8 and VEGF secretion renders melanoma cells which are considered as a possible escape mechanism in chemotherapy and results in resistance against the cytotoxic effects of the drug. These side effects might conduct the cells to more aggressive phenotype, and metastatic capabilities 5.
A major limitation of cancer chemotherapy is the inability to escalate doses of an anticancer drug due to intolerable cytotoxic side effects. This limitation can be overcome by using a combination of natural based drugs administered at lower dosage 22. The most widely used chemotherapeutic combination therapy includes application of cisplatin, vinblastine and dacarbazine 23. Doxorubicin (DOX) is a type of chemotherapeutic agent which is commonly used to treat a wide range of cancers. Nevertheless, DOX induced severe toxicity and undesirable side effects. Oktem et al planned a study to determine the combination of resveratrol as a potent antioxidant and DOX to reduce the toxic effects of cellular damage caused by doxorubicin 24.
Aloe-emodin (AE) is a natural hydroxyanthraqui-none compound of Aloe barbadensis Miller (Aloe vera L.) which has been known as an effective anticancer agent in traditional medicines. Tabolacci et al designed a study to investigate the antineoplastic effect of AE on B16–F10 melanoma cells and their results showed the anti-proliferative and anti-metastatic properties of Aloe-emodin against mouse metastatic melanoma cells 25.
In 2013, Zhang et al showed that Trametes robiniophila Murrill (Huaier) aqueous extract can be used as a promising complementary agent against melanoma cancer. Huaier has been well-known in Traditional Chinese Medicine (TCM) as an anti-cancer agent which exerted cytotoxic effect against melanoma via inhibition of cell proliferation and induction of apoptosis through the mitochondrial pathway 26. Green tea is an evergreen plant that possesses antioxidant and pro-oxidant activities, so that it is recognized as a cancer preventive agent. In 2012, Fujiki et al evaluated the synergistic effect of chemotherapeutic drugs with green tea catechin and approved synergistic treatment with this natural extract and indicated that anti-cancer drugs induced apoptotic cell death, prevented tumor formation and proliferation 27.
Marine-derived natural products such as saponins represent therapeutic properties to eradicate cancer. Accordingly, recent studies have gained an increasing attention towards finding and producing novel natural drugs from marine organism as a potential source of anticancer drugs 28. In case of improving the effectiveness of chemotherapeutic strategies, reducing the dosage of anticancer drugs is considered appreciable in minimizing adverse side effects 20.
Jaspine B is a natural product which is isolated from the marine sponges Pachastrissa sp. and Jaspis sp. Salma et al reported that Jaspine B acts as apoptosis inducer and its cytotoxic effect is produced via caspase-dependent pathway against murine and human melanoma cells 29.
In previous research, a study was designed to isolate and evaluate the cytotoxic effect of Persian Gulf brittle star (Ophiocoma erinaceus) saponin and revealed that the extracted saponin exerted hemolytic and cytotoxic effect on cervical carcinoma cells 30. Furthermore, the anti-proliferative properties and effects of brittle star dichloromethane extract on B16F10 melanoma cells were evaluated and it was indicated that marine echinoderm extracts can exert their cytotoxicity on melanoma cells via apoptosis recruitment 31. Li et al examined the sea cucumber Holothuria nobilis Selenka saponin, echinoside A, as a significant anticancer agent in prostate carcinoma cells and their results showed that echinoside A could inhibit Topoisomerase2 (Top2) and decrease the tumor growth suggesting anti-cancer potential of marine saponins in cancer therapies 32. In another study, it was demonstrated that saponin extracted from sea cucumber (H. leucospilota) has potent hemolytic and cytotoxic effect against lung cancer cells so these compounds may present a great opportunity to be used as effective anti-cancer agents 33.
Consequently, combination therapies based on natural products and approved drugs, such as the combination of sea cucumber saponin and dacarbazine can be proposed as novel chemotherapeutic modality against melanoma. Furthermore, future studies are required about the detailed mechanisms involved in apoptosis pathway induced by sea cucumber saponin and dacarbazine.
Conclusion :
Our results clearly indicated that sea cucumber saponin and dacarbazine exerted cytotoxicity on melanoma cells through apoptosis recruitment and administration of saponin extracted from sea cucumber increases the sensitivity of melanoma cells to chemotherapeutic drug, exhibits the strongest anti-cancer effect compared with dacarbazine and may provide a promising new therapeutic approach to melanoma. In addition, dacarbazine in combination treatment with sea cucumber saponin render anti-cancer properties via intrinsic apoptotic pathway which was confirmed by activation of caspase -3,-9. These outcomes represent reliable evidence supporting the application of sea cucumber saponin as an adjunct to dacarbazine in advancement of effective therapy in melanoma.
Acknowledgement :
The authors would like to thank Research Center of Applied Biology, Mashhad Islamic Azad University.
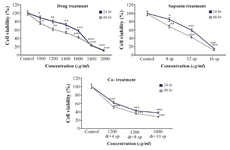
Figure 1. Dose dependent effects of sea cucumber saponin, dacarbazine alone and in combination on cell viability in B16F10 melanoma cells 24, 48 hr. As shown, in combination treatment, IC50 value obtained in low toxic concentration of each treatment alone demonstrating potent activity of combination therapy on melanoma cell viability (sp=saponin, dr=dacarbazine).
*p<0.05, **p<0.01 and ***p<0.001 were considered significant between control and experimental groups.
|
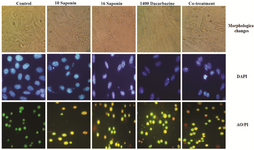
Figure 2. Effect of sea cucumber saponin and dacarbazine on cytomorphological changes of B16F10 cells. Phtomicrographs indicated that B16F10 cells were treated with IC50 concentrations of sea cucumber saponin, dacarbazine and co-treatment stained with DAPI and AO/PI mixed dye administrated apoptosis cell death (sp=saponin, dr=dacarbazine, Co- treatment=1200 µg/ml dacarbazine+4 µg/ml saponin).
|
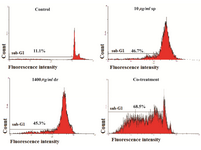
Figure 3. Apoptosis detection by flow cytometry in B16F10 cells, Flow cytometry histogram of un-treated and treated B16F10 cells with IC50 concentration of sea cucumber saponin, dacarbazine exhibited increase in sub-G1 region demonstrating mediation of an apoptotic cell death in cytotoxicity of sea cucumber saponin, dacarbazine and co-treatment (sp=saponin, dr=dacarbazine, Co-treatment=1200 µg/ml dacarbazine+4 µg/ml saponin).
|

Figure 4. Effect of sea cucumber extracted saponin and dacarbazine on caspase -3 and caspase -9 activity. As shown, incubation of B16-F10 cells with increasing concentration of sea cucumber saponin and dacarbazine were enhanced significantly 48 hr after treatment, in addition more caspase activation resulted in combination treatment groups. p<0.01, p<0.001 were considered significant between experimental groups and control.
|
|