Distribution and Diversity of hmw1A Among Invasive Nontypeable Haemophilus influenzae Isolates in Iran
-
 Siadat, Seyed Davar
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953311, E-mail: d.siadat@gmail.com
Siadat, Seyed Davar
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran, Tel: +98 21 66953311, E-mail: d.siadat@gmail.com
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Vaziri, Farzam
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Davari, Mehdi
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Fateh, Abolfazl
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Pourazar, Shahin
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Abdolrahimi, Farid
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
-
Ghazanfari, Morteza
-
Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran
Abstract: Background: The pathogenesis of nontypeable Haemophilus influenzae (NTHi) begins with adhesion to the rhinopharyngeal mucosa. Almost 38-80% of NTHi clinical isolates produce proteins that belong to the High Molecular Weight (HMW) family of adhesins, which are believed to facilitate colonization.
Methods: In the present study, the prevalence of hmwA, which encodes the HMW adhesin, was determined for a collection of 32 NTHi isolates. Restriction Fragment Length Polymorphism (RFLP) was performed to advance our understanding of hmwA binding sequence diversity.
Results: The results demonstrated that hmwA was detected in 61% of NTHi isolates. According to RFLP, isolates were divided into three groups.
Conclusion: Based on these observations, it is hypothesized that some strains of nontypeable Haemophilus influenzae infect some specific areas more than other parts.
Introduction :
Nontypeable Haemophilus influenzae (NTHi) is a small, fastidious gram-negative coccobacillus that colonizes the human pharynx, the only known natural reservoir. Although generally considered a commensal, NTHi is capable of inducing localized infections of the upper and lower respiratory tracts, such as acute otitis media, sinusitis and bronchitis, as well as, more rarely, severe invasive infections such as pneumonia, bacteremia, and meningitis 1-3.
The initial step in the pathogenesis of NTHi involves establishment of bacteria on the rhinopharyngeal respiratory mucosa followed by contiguous spreading within the respiratory tract and, occasionally, to sterile sites 4.
High Molecular Weight (HMW) adhesins, which have been reported in 38-80% of NTHi isolates, are the major adhesins responsible for attachment to human epithelial cells 5. The HMW adhesins are a family of paralogous proteins encoded by the hmw locus, which is present in two complete copies, hmw1 and hmw2, located discontiguously on the NTHi chromosome 6. Each hmw locus encodes three proteins, hmwA, hmwB, and hmwC and both the gene content and the chromosomal locations of the hmw loci are conserved across isolates 7,8.
The hmwA encodes the functional HMW adhesins while hmwB and hmwC encode proteins required for maturation, glycosylation and secretion of mature HMW adhesions 9,10. In particular, the 124 amino acids between residues 114 and 237 in mature HMW1 have been found to be essential for full-level adhesive activity 11. These essential regions have been previously referred to as HMW1 core-binding domains and the encoding sequences as the hmw1A core-binding domain sequences 12,13. Amino acid diversity within the HMW binding domain likely serves the diversity of hmwA locus in various strains 14.
The research presented in this study identified diversity of the hmw1A locus in different clinical strains isolated from nasopharynx, middle ear, pleura and conjunctiva.
Materials and Methods :
Bacterial strains and growth: Nontypeable Haemophilus influenzae (NTHi) strains were obtained from four infection sites: nasopharyngeal or throat isolates from healthy children, strains cultured from middle ear aspirates from children with otitis media and pleural fluid and conjunctiva isolates obtained from children with invasive diseases. Strains were isolated between 2011 and 2013, at different locations in Iran. NTHi isolates were grown overnight on 7% chocolate agar plates supplemented with Vitox (Oxoid Ltd., Basingstoke, Hampshire) at 37°C in 5% CO2 and were identified by their requirements for X and V factors and by their lack of reaction with antisera against capsular (Phadebact; KaroBio Diagnostics AB). For DNA extraction, bacterial strains were grown in Haemophilus Test Medium (HTM) broth, consisting of Muller-Hinton broth (Oxoid Ltd.) supplemented with 0.5% yeast extract and HTM supplement (Oxoid Ltd.), and incubated under the same conditions.
DNA amplification: Oligonucleotide primers were designed (Table 1) on the basis of the sequence of the hmw1A gene. Regions with low variability were chosen. The sequence of each primer was checked for homology to other sequences that may also be amplified by them, in the GenBank and EMBL databases. The PCR product was a 1.045-bp nucleotide fragment containing regions with low variability of the hmw1A gene.
Restriction fragment length analysis: The amplified DNA fragments were digested with one restriction endonuclease (Taq1) (5'TCGA-3'AG CT), which was selected on the basis of the nucleotide sequence of the hmw1A gene of NTHi.
Nucleotide sequencing: A PCR product of each restriction fragment pattern (rfp) was randomly chosen and was sequenced.
Sequence analysis: Sequence analysis was performed with the BioEdit and CLC Main Workbench 5 software.
Results :
The strains containing hmw1A gene were separated with PCR of hmw1a gene. Only 20 of the 32 isolates contained the hmw1a gene. Polymorphism in the hmw1A gene was found in Haemophilus influenzae strains isolated from the nasopharynx, middle ear, pleural fluid and conjunctiva in Iran. Figure 1 shows the RFLP patterns of hmw1A gene in nontypeable Haemophilus influenzae strains isolated from the nasopharynx, middle ear, pleural fluid and conjunctiva by Taq1 digested PCR products.
This restriction enzyme (Taq1) gave three different patterns with all isolated strains, isolated strains from pleural fluid and conjunctiva contained restriction fragment pattern (rfp1) and isolated strains from naso-pharynx contained rfp1 and rfp2 (Figure 2).
A sample was selected randomly from each pattern for sequence. The PCR product of the numbers (Ppn) 3, 9 and 13 were representative of the restriction fragment pattern of 1, 2 and 3, respectively. CLC Main Workbench 5 software revealed that sample numbers 1, 2 and 3 were similar to strains of nontypeable Haemo-philus influenzae with GenBank accession numbers (Gan), AY497551, HIU08876 and AY497554, respectively (Figure 3).
Discussion :
Nontypeable Haemophilus influenzae is a major cause of localized respiratory tract diseases, including otitis media, sinusitis, conjunctivitis, bronchitis and pneumonia and strains of bacteria are various 5,15,16. For prevention of infection or immunomodulation of chronically infected individuals for appropriate humoral and cellular immune mechanisms, it is necessary to recognize the available strains in different regions.
In this study, an attempt was made to determine the prevalence of hmwA in a collection of 32 NTHi isolates collected between 2011 and 2013 from the throat isolates of healthy children, the middle ears of children with otitis media and pleural fluid and conjunctiva isolates obtained from children with invasive diseases. The hmwA prevalence (63%) in this isolate collection was consistent with previous studies, which report prevalences ranging from 38 to 80% 5,8,17. In previous studies, to determine the type of strains in a region, some techniques such as MLST were used, or genome of all strains had been sequenced 12.
In this study, it was revealed that hmw1A genes of nontypeable Haemophilus influenzae in Iran are similar to strains of nontypeable Haemophilus influenzae with GenBank accession numbers, AY497551, HIU08876 and AY497554. Several studies have demonstrated that phase variable HMW adhesin expression has important implications for NTHi pathogenesis 18-20. In this study, it was found that the restriction fragment pattern 3 (rfp 3) that is similar to GenBank accession number HIU08876 is not indeed in throat isolates but is present in some otitis isolates and rfp 1 that is similar to GenBank accession numberAY497551 is present in pleural fluid, throat, conjunctiva and otitis isolates. Efficient adherence of nontypeable Haemophilus influenzae to a variety of mammalian cells is dependent on bacterial expression of HMW proteins 21 and based on these observations, it is hypothesized that some strains of nontypeable Haemophilus influenzae infect some specific areas more than other parts or in other words, the strains on those areas are more connected and they stimulate colonization. However, more studies are needed to prove this hypothesis. To obtain and submit a comprehensive report, it is required to collect more samples and apply newer molecular methods.
Finally, this work has important public health implications since the HMW adhesins have been proposed as potential components of the NTHi targeted vaccine. Phylogenetic analysis can be used to inform the selection of HMW adhesins for inclusion in a vaccine, e.g., selecting representative alleles from each phylogenetic cluster. In previous studies, the extensive amino acid diversity and the lack of disease-specific hmwA sequence clusters underscore the challenges of targeting HMW adhesins as vaccine components 2 but in this study, specific sequences of hmwA in the specific sites were observed.
Conclusion :
The prevalence of hmw1A among invasive nontypeable Haemophilus influenzae isolates in Iran is almost similar to previous studies reported in other countries, and based on our observations, strains with specific sequence of hmw1A in some parts of the body were found, whereas in other parts of the body other sequences of the hmw1A were observed.
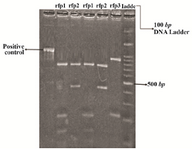
Figure 1. Restriction fragment pattern (rfp) of hmw1A gene fragment amplified by PCR. RFLP patterns were obtained after digestion of PCR products with Taq1. Number 0, PCR products of hmw1A gene without restriction enzyme; numbers 1-3, digestion products of hmwA gene fragments amplified (three different patterns); the last lane, molecular weight marker.
|
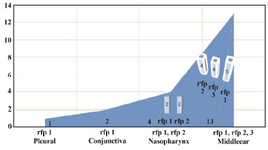
Figure 2. This chart describes the number and type of pattern of the strain isolated from each section.
|

Figure 3. Strains with a coefficient of similarity value≥80% were considered to belong to the same cluster. On the right, the strain code number and the different clusters (clusters 1 through 3) are reported.
|

Table 1. Polymerase Chain Reaction (PCR) primers used in this study
|
|