Interferences in the Optimization of the MTT Assay for Viability Estimation of Proteus mirabilis
-
Grela, Ewa
-
Department of Bioorganic Chemistry, Faculty of Chemistry, Wrocław University of Technology, Wroclaw, Poland
-
Ząbek, Adam
-
Department of Bioorganic Chemistry, Faculty of Chemistry, Wrocław University of Technology, Wroclaw, Poland
-
 Grabowiecka, Agnieszka
Wrocław University of Technology, Wybrzeze Wyspianskiego Wroclaw, Poland, Tel: +48 71 320 24 27 E-mail: agnieszka.grabowiecka@pwr.edu.pl
Grabowiecka, Agnieszka
Wrocław University of Technology, Wybrzeze Wyspianskiego Wroclaw, Poland, Tel: +48 71 320 24 27 E-mail: agnieszka.grabowiecka@pwr.edu.pl
-
Department of Bioorganic Chemistry, Faculty of Chemistry, Wrocław University of Technology, Wroclaw, Poland
Abstract: Background: The chromogenic assay based on MTT bioreduction was adapted to Proteus mirabilis viability estimations. We primarily intended to use the assay for the evaluation of novel antimicrobial compounds, including structures with possible permeabilizing activity. Therefore, the influence of basic permeabilizing agents like Triton X-100 and EDTA upon the MTT assay was studied.
Methods: 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) was used as a substrate for the whole-cell dehydrogenase activity estimations. The amount of formazan product was evaluated in the end-point reactions terminated with acidic isopropanol or in the continuous reactions run in the presence of low detergent concentrations.
Results: The generally established procedure of the end product dissolution with acidic isopropanol caused absorbance instability which strongly affected the results accuracy. The disadvantage was especially pronounced when the assay was conducted in Mueller-Hinton Broth. PBS with 0.01% Triton X-100 used as the reaction medium allowed to omit the formazan dissolution step and follow the microbial MTT reduction in a continuous mode. It was observed that in Proteus mirabilis with a compromised outer membrane the assay score was artificially increased above the untreated control.
Conclusion: The dependence of the assay results on the cell integrity might be a major drawback of the MTT assay application for the evaluation of novel antimicrobials against Gram-negative microorganisms. On the other hand, the MTT reduction could be conveniently used to assay the permeabilization degree in biotechnological protocols.
Introduction :
In every field of microbiological research, biotechnological as well as medical, routine practices are followed to determine the bacterial culture viability. Favorable laboratory techniques should allow for relatively fast and convenient processing of a large number of samples, without long incubation times and multi-step protocols. The traditional manual, spread plate method, though practicable in every laboratory, even in less well-equipped ones, is time-consuming and does not allow for immediate evaluation. The proper performing of colony counts requires certainty in laboratory practice and does not work for cells that tend to agglomerate in suspensions. Moreover, swarming ability of many bacterial species makes accurate colony counting difficult even on specialized media. The optical density of a culture reflects neither the metabolic status nor the viability of the microorganism. Modern methods based on cell structures, radio- and fluorolabeling, are extremely valuable tools that provide a variety of information, but they are costly and involve the use of hazardous chemicals. Colorimetric assessment of metabolic efficiency of cells provides an attractive compromise between the need for accuracy and simplicity of a method, especially when optimized for microplates and automation.
Currently, chromogenic assays based on tetrazolium dyes are becoming more and more popular. These tests allow for the indirect estimation of cells' viability via their redox activity 1. The assay protocols depend strongly on the type of tetrazolium salt used as a substrate of the enzymatic reaction. Two basic classes of tetrazolium salts can be distinguished. The first group is cell-impermeable, and usually an additional electron mediator is necessary. Salts like XTT, WST-1, WST-8, etc. can be included in this class. Their reduced form is water-soluble and no solvent is needed to evaluate results by spectrophotometric methods. These dyes offer the advantage of continuous dehydrogenase assay but possible cytotoxic effect of the electron mediator was reported and must be taken into consideration 2.
Tetrazolium dyes which do not require external electron mediator and yield water-insoluble formazans are represented by MTT 3 described for the first time by Mosmann 4. MTT (3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) has numerous applications in microbiology. It is used mainly for spectrophotometric assessment of the metabolic activity of microorganisms 5. Due to the color yielded, the enzymatic MTT reduction can also be used for microscopic observations of living cells 6 or even biofilm quantification 3,7. The possibility of using microplates and automated reading adapts the assay for simultaneous examination of numerous samples and significantly improves the test in terms of time and cost 8. These advantages contribute to expansion of the MTT assay in microbiological laboratories and creation of various test modifications 9-13. The lack of standardization, however, is a strong argument against the method of reducing tetrazolium salts for clinical research 1,14. Another considerable disadvantage of MTT salt is the formazan insolubility in water. This feature strongly limits its applications in comparison with tetrazole substrates, which give a water-soluble product. The necessity to dissolve the precipitate before absorbance measurement makes MTT bioreduction an end-point assay 15. Moreover, reduced MTT forms large intracellular deposits, capable of damaging living cells during tests. It is another reason why application of the MTT test in clinical cytotoxicity estimations is limited and generally criticized 16,17.
The MTT-formazan crystals are soluble in a variety of common organic solvents like isopropanol, DMSO or even mineral oil 5,8. Despite the lack of standardized protocol and various interferences reported by researchers, it has been generally recognized that absorbance of dissolved formazan in a bacterial culture sample recorded near 550 nm is directly proportional to the number of metabolically active cells 18. Furthermore, the concentration of the substrate does not interfere with measurement of the product under proper test conditions. Therefore, the MTT assay is generally considered a comparatively fast method for evaluating the efficiency of antimicrobial agents 19.
Our main research interest was studying the in vitro activity of urease inhibitors against ureolytic bacterial strains. This involved the assessment of their possible bacteriostatic or bactericidal effect. The aim of this work was to verify the applicability of the MTT assay for the viability evaluation of urinary tract pathogen Proteus mirabilis (P. mirabilis). Currently, limited information is available on microbiological applications of MTT compared to eukaryotic studies 4. The assay seemed an alternative to other ways of Proteus cell number determination which are strongly prone to inaccurateness. P. mirabilis is capable of morphological transformations into cell forms differing strongly not only in motility but also cell size 20. It results in difficulties in the use of most common techniques including any based on optical density measurements (like broth dilution MIC estimations) or plate count methods. Additionally, Proteus tends to form biofilms even during short incubations in contact with plastic surfaces, which interferes with spectrophotometric reading of microplates. Release of cell-surface-bound formazan crystals with organic solvent might result in biofilm disintegration and improve reliability of obtained data.
Materials and Methods :
Chemicals: 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), MTT formazan, Triton X-100, etylenediaminetetraacetic acid (EDTA) were obtained from Sigma-Aldrich, Poland. Organic solvents came from POCh, Poland. All chemicals were of analytical purity grade and did not undergo further purifications.
Microorganism and growth conditions: P. mirabilis PCM 543 was purchased from the Polish Collection of Microorganisms (Wrocław, Poland). The strain was routinely maintained on Mueller-Hinton 2 Agar (Biocorp, Poland) at 37°C. Cells used in all described experiments were collected from overnight cultures in Mueller-Hinton 2 Broth (Biocorp, Poland) grown at 120 rpm, 37°C.
Preparation of reagents solutions: For a routine assay, MTT stock solution was prepared as 5 mg mL-1 tetrazole in phosphate buffered saline (PBS, 10 mM, pH=7.2), sterilized by filtration. The solution was stored refrigerated for maximum two weeks. Microbiological contaminations during storing could be easily detected as purple formazan precipitate. Acidic isopropanol was prepared as a 1.5% (v/v) solution of hydrochloric acid in isopropanol and stored in glass.
0.5 McFarland Standard was prepared by adding 0.5 ml of 0.048 M BaCl2 to 99.5 ml of 0.18 M H2SO4 with constant stirring. The proper density value between 0.08 and 0.10 of the turbidity standard was verified by OD650 measurements. The suspension was stored in darkness for no longer than a month.
Phosphate buffered saline was prepared as 10 mM KH2PO4/Na2HPO4 x7H2O pH=7.2 with 15 mM NaCl and 0.2 mM KCl.
MTT assay conditions: The colorimetric test was conducted as a microassay using sterile Eppendorf 96/F-PP microplates with lids. Polypropylene plates were chosen to reduce biofilm formation. This particular Eppendorf brand is characterized with clear well bottom which enabled direct reading of the plates after test termination. Next, 10 µl of MTT solution in PBS was added into the well containing 90 µl of bacterial cell suspension in Mueller-Hinton Broth or PBS. For the standard MTT assay, 107-108 CFU ml-1 was used. Cells’ density ranged from 106 to 1012 CFU ml-1. Under standard assay conditions, mixtures were incubated for one hour at 37°C on the incubated ELMI DTS-4 SkyLine orbitary shaker at 300 rpm. 100 µl of acidic isopropanol was then added directly into the reaction mixture (without aspiration of wells) and plates were further incubated for an hour to allow solubilization of crystallized formazan. Plates were read at 550 nm or scanned between 400 and 700 nm with TECAN-Sunrise absorbance reader equipped with a gradient filter and Magellan software.
Effect of permeabilizing agents: To study the effect of permeabilizing agents, MTT assay was conducted in the presence of 0.01-1% Triton X-100 and 0.003-1 mM EDTA. EDTA was prepared as 10 mM stock solution with pH adjusted to neutral. Permeabilizers were added at the start of MTT assay incubations without preincubation step. Negative control wells contained sterile Mueller-Hinton Broth or PBS with complete range of permeabilizers’ concentrations. pH of reaction mixtures was checked prior to and after incubation. Triton X-100 was used either in typical end-point assay terminated with acidic isopropanol or in continuous assay in which absorbance was followed over time without dissolution step. Triton X-100 was also used as an additive in acidic isopropanol in 0.01-1%.
Cell disruption conditions: Dense bacterial culture (above 109 CFU ml-1) was centrifuged for 2 min at 5000 g and washed with PBS. After the second centrifugation, a bacterial pellet was suspended in 10 mM PBS with addition of 50 mM sodium sulfite. The suspension was sonicated with 10 cycles of 30 s pulses followed by 2 min cooling on ice. After each sonication cycle, aliquots were collected. Bradford assay was used to determine protein quantity. The samples were then centrifuged and MTT was conducted separately on supernatant and cell debris sediment resuspended in buffer.
CFU determination: Buffered Peptone Water was used as a medium for culture serial dilutions. 100 µl samples of bacterial suspensions were spread either over CLAD agar or Plate Count Agar (Biocorp, Poland) and a drop of 95% ethanol was added to the plate cover to prevent swarming 20. Colonies were enumerated after 48 hr incubation at 37°C.
Adjusting bacterial cell number: As the size of a Proteus mirabilis cell may change dramatically depending on growth conditions which strongly influence optical density/cell number ratio, actual number of cells in suspensions routinely adjusted to optical density of 0.5 McFarland Standard was determined using standard curve previously prepared from CFU counts of 18 hr (mid-log) liquid culture.
Cell-produced formazan isolation for dilution experiments: Undiluted overnight bacterial culture (above 109 CFU ml-1) was used to produce formazan crystals. MTT solution was added to the culture to a final concentration of 0.5 mg ml-1 and incubated for several hours to obtain a nearly black, thick suspension. Samples were gently centrifuged. In further tests, both sediment and supernatant aliquots were used.
Results :
Some recent papers describing the optimization of the MTT assay concern modifications in the final step and suggest using DMSO to improve formazan dissolution for absorbance readings 12. This requires separation of cells with bound formazan crystals from the MTT reaction medium. In the case of P. mirabilis PCM 543, the crystallized product could be observed not only in a cell-bound form but also free in the reaction medium. This forced the choice of dissolving the product directly in the incubation mixture (Figure 1). It was decided to follow Mosmann's 4 original reports and arbitrarily apply acidic isopropanol as a solving agent. This allowed us to avoid possible inaccuracies arising from plate centrifuging and wells’ aspiration, and to exclude harmful dimethyl oxide from the protocol. First, the proper range for the bacterial cell number per assay that would yield proportional amount of formazan was standardized. 1010 CFU ml-1 was defined as the maximum Proteus cell number in 1 hr assay in PBS and Mueller-Hinton Broth with 0.5 mg ml-1 MTT (substrate concentration based on literature reports) 12.
It was observed that acidified isopropanol used to terminate the assay caused absorbance changes that continuously increased with time. Thus, the proper dissolving time that would allow reproducibility of readings was impossible to determine. The degree of absorbance instability was dependent on the assay medium and the amount of cells. Besides the final assay product, the MTT assay mixture contained several components that absorbed between 550 and 650 nm and changed quantitatively during both the reduction and dissolution steps. As the observed alterations at 550 nm might derive from peak absorbance shifts, 400-700 nm spectra were recorded over time in Mueller-Hinton Broth and PBS for two Proteus suspensions differing in cell number by an order of magnitude. As the reference, spectra of formazan crystal suspension separated form bacterial culture by centrifugation were made, which were characterized by the broad plateau between 490 and 640 nm with a small absorbance peak at 620 nm. It was assumed that absorbance changes at 550 nm resulted not solely from formazan crystal dissolution, but also from the isopropanol effect upon the bacterial cells and media components. At 620 nm, most likely formazan crystals, both free and cell-bound, were detected, while at 650 nm, simply cell density was read.
When 108 CFU was introduced into the assay mixture in the Mueller-Hinton Broth (starting OD650=0.08), a progressive increase in the absorption spectra was observed over the entire 90 min of MTT reduction. A marked peak between 650-670 nm was visible which most likely reflected the continuous and gradual slowing-down of cell proliferation. When acidified isopropanol was added, the spectrum narrowed and the maximum shifted as expected to a single intense peak at 550 nm. The effect of organic solvent, however, was highly unstable. After the initial jump by DA550=1.3, the absorbance kept decreasing for the next two hours of recording, at a rate of 0.012 AU min-1. A650 fell off sharply upon the addition of the solvent. Readings at 620 nm began to decrease constantly at the rate of approximately 0.0026 AU min-1 (Figures 2A and 2B). The intensity of the A550 rose upon addition of the solvent and the rate of subsequent decrease was dependent on the amount of reduced product as shown by monitoring assays carried out at varying MTT concentrations (Figure 3).
In PBS, recorded scan lines rose uniformly for the first 20 min of the MTT assay, and then became stable for the remaining assay time; obviously, cells transferred into the buffer lost their proliferation potential. A broad plateau was noticeable with slight maxima at 560 and 610 nm. Upon isopropanol addition, a single peak formed as readings dropped in the whole spectral range, with most significant decrease at wavelengths above 560 nm (Figure 4A). No absorbance jump around 550 nm was observed. Instead, after an initial rapid decrease, the final reading was nearly stable with slight changes of about 0.46*10-3 AU min-1 at 550 nm and 0.335*10-3 AU min-1 at 620 nm (Figure 4B).
When the starting cell number was higher by an order of magnitude under the same assay conditions, readings prior to isopropanol addition reached plateau in PBS as well as Mueller-Hinton Broth within 30 min of incubation with MTT. Though no sudden absorbance rise occurred at 550 nm, either in the buffer or the growth medium after solvent incorporation in these stationary assays, the kinetics of consequent absorbance decrease was again different. Similarly as described above, after a sudden drop, the decrease rate in PBS stabilized at 0.005 AU min-1 and 0.0026 AU min-1 for A550 and A620, respectively. In Mueller-Hinton Broth the decrease rate at 550 nm was constant and around 0.016 AU min-1 while A620 initially fell down to reach the decrease rate of 0.0073 AU min-1.
Subsequently, the individual response of the reaction mixture components to acidic isopropanol treatment was analyzed. Uninoculated reaction mixtures in Mueller-Hinton Broth were characterized with absorbance rise by around 0.2 units at 550 nm after solvent addition. In original Mueller-Hinton Broth not containing MTT, the addition of acidic isopropanol caused similar A550 reading increase (Figure 3). This ruled out the assumption of noncellular reduction of MTT promoted by Mueller-Hinton Broth components and pointed to certain instability of the medium under the assay conditions. To exclude cell contribution to absorbance changes at 550 nm during the dissolution step, assay mixtures were mimicked by suspending commercially obtained formazan crystals in PBS and Mueller-Hinton Broth. Concentration of 0.5 mg ml-1 was used, which corresponded to the maximum amount of product that could be generated microbiologically under standard assay conditions. In PBS, after acidic isopropanol incorporation, A550 was stable for at least 60 min, while in Mueller-Hinton Broth, the absorbance began to decrease 10 min after solvent addition (Table 1). The absorbance instability in MHB was not pH dependent as both media responded to added HCl with similar acidification; moreover, formazan solubiliz-ation using isopropanol without HCl at pH=7.5 gave similar results (Table 1).
In gram negative microorganisms, dissolution of crystals displayed on the cell surface or formed in the periplasm must be associated with changes in the outer membrane integrity. Thus, the presence of substances that affect cell surface (chelators, detergents) may strongly influence the MTT assay and lead to artifacts. This was our concern as some of the new compounds planned to verify using the MTT method bear structural detergent-like features. On the other hand, it was reported that nonionic detergent, Triton X-100, at 1% concentration allowed for the monitoring of Eschericia coli (E. coli) MTT reduction in a continuous mode without the need to solubilize the product. This was due to stable formazan microemulsion formation that could be measured spectrophotometrically 21. Considering the absorbance instability of isopropanol dissolved product, it was decided to try a similar approach for P. mirabilis.
A range of Triton X-100 or EDTA concentrations was included in P. mirabilis PCM 543 assays, and their effect upon the yield of formazan, crystals dissolution, absorbance stability and the strain viability was investigated. Thus, absorbance scans of the assays in PBS containing Triton X-100 were performed. Prior solubilization and detergent concentration of 0.01% resulted in slightly higher reading at 550 nm than the control without the additive. After application of acidic isopropanol, the absorbance reading of the control and the assay with detergent brought similar results. Triton concentrations above 0.1% caused a decrease in readings and the obtained lines were similar to solubili-zation step though organic solvent was not used (Figures 5A and 5B).
In Mueller-Hinton Broth, 0.01% Triton X-100 caused significant reading increase at 550 nm before and after acid isopropanol addition compared to the control. The scan characteristics and the test effectiveness at concentrations above 0.1% was the same as in PBS. Plate counts using diluted aliquots of assay mixtures showed a slight decrease in cell number from 8 to 12% after 60 min of incubation in the presence of 0.1% to 1% Triton X-100.
In PBS containing 1 mM EDTA, the overall test result read at 550 nm was by 50% higher before and after the solubilization step compared to the control. The addition of acidic isopropanol influenced the scan shape, which narrowed to a clear peak, but the intensity of absorbance remained unchanged. EDTA at 0.1 mM concentration did not influence the test result. In Mueller-Hinton Broth, the effect of 1 mM EDTA on the Proteus MTT test was negative. The spread plate CFU counts showed significant reduction of number of cells which are able to divide after incubation, with EDTA concentrations exceeding 0.03 mM. In the flow cytometry analysis of the samples, populations of cells with low granularity were present (data not shown). Worryingly, the MTT result was not proportional to the cell viability loss; contrarily, the presence of 0.03 mM EDTA increased the assay result by 15% compared to the control (Figure 6).
The disparate evaluation of Proteus metabolic status assayed by the MTT test and the growth method after EDTA treatment prompted us to follow MTT reduction activity in P. mirabilis cells exposed to sonication. The purpose was to investigate how cell envelope injury would affect the assay score. The MTT test run using samples of crude extract supernatant and cell debris resuspended in buffer gave the highest result after 2nd cycle of sonication, when significant protein release was obtained due to outer membrane disintegration. The MTT reduction could be still detected in assays performed with samples subjected to several subsequent sonication pulses resulting in increasing protein content (cell disruption extent) (Figure 7).
Based on the obtained results, 0.01% Triton X-100 was chosen as the additive that might improve MTT assay of P. mirabilis PCM 543. Assays containing 0.01 and 0.05% detergent were followed at A550 in a continuous mode. The parallel control without the detergent, in which the amount of formazan produced was quantified in an end-point mode with acidic isopropanol was used for comparison (Figure 8). The kinetics of the continuous assays was characterized with more accurate course compared to the control. The final results after 60 min of incubation were similar, but the presence of detergent significantly accelerated cell saturation in a concentration dependent manner.
As mentioned before, the main disadvantage of using organic solvent as the dissolving agent was the absorbance instability. Thus, 0.01 to 0.5% Triton X-100 was also evaluated as an additive to acidic isopropanol. Detergent enriched solvent was introduced into suspensions of commercially obtained formazan crystals in PBS and Mueller-Hinton Broth. Absorbance at 550 nm was followed in 20 min intervals. The results listed in table 1 express the percentage of absorbance change taking T0 absorbance as 100%. Formazan solution in culture medium was obtained using isopropanol or its acidified equivalent was formed rapidly but provided stable absorbance readings only for up to 20 min. Upon prolonged incubation, absorbance began to gradually decrease. Formazan dissolution in PBS was slower-isopropanol or acidified isopropanol addition resulted in increase of absorbance over the first 20 min, then it remained considerably time-stable. The presence of detergent in organic solvent stabilized absorbance readings, but the absorbance values at 550 nm suggested less efficient solubilization. When Triton X-100 itself without organic solvent was added to formazan crystal suspensions in Mueller-Hinton Broth or PBS, the obtained absorbance values were no more than 20% compared to acidic isopropanol mediated solubilization (data not shown). This was in contrast to results described above when Triton X-100 was present during microbial formazan crystals formation either in the continuous or the end-point assay.
Discussion :
Colorimetric assessment of bacterial metabolic activity via a single substrate assay is unquestionably an attractive alternative to other techniques. Bacterial viability per se is a multi-aspect phenomenon, as the cell may temporarily or irreversibly enter diverse physiological states in response to the environment, and the argument continues in which the indicator of the cell condition can be a rational measure of the susceptibility to exogenous agents. Bacterial culture is a mixed population diverse in ability to grow, cellular integrity and level of metabolic activity. The quantification of the latter as the potential to reduce tetrazole salts via redox enzymes has long been proposed. It was reported that the amount of produced formazan was directly proportional to the number of viable cells, considering viability as culturability 22. It was tried to adapt MTT reduction for P. mirabilis susceptibility testing using acidic isopropanol as the solvent. As the product is measured at 550 nm, several factors had to be considered that might influence the test result. Bacterial cell suspension may increase in density if the assay is conducted in a growth medium, and then partially disintegrate upon organic solvent addition. The cells change their spectral properties when crystals are formed, part of the crystallized formazan may be free in assay medium, and finally the medium may change clarity during the solubilization step. In P. mirabilis assays, formazan dissolution proceeded differently depending on the reaction medium. The cells used in each assay were generated in Mueller-Hinton Broth and washed before transfer into assay mixture. Thus, the observed differences were not caused by secondary metabolites or pH changes deriving from old culture medium residue. The underlying reason had to be MTT reduction process itself, most likely the amount and cellular localization of formazan crystals, along with susceptibility of the outer membrane to solvent action. The observed absorbance instability in Mueller-Hinton Broth was truly discouraging, as this medium is the recommended standard for bacterial susceptibility testing. The assays conducted in buffered saline gave the most credible results. The cell suspension did not increase in density during tests and the absorbance after the dissolution step became significantly more stable. The buffer did not become cloudy upon isopropanol addition, contrary to the growth medium. Moreover, a combination of phosphate salts and organic solvent is a popular outer membrane weakening mixture, used for example in the periplasmic enzymes extraction. Thus, PBS may be a favorable environment for outer membrane breakage and crystal dissolution with isopropanol. When permeabilizing agents were tested, low concentration of nonionic detergent (0.01% Triton X-100) incorporated at the beginning of the assay allowed us to follow the reduction reaction in a continuous way without the need to use an organic solvent. Concentrations above 0.5% reduced test results and CFU counts of reaction mixtures were lowered by 10%. Triton X-100 addition after incubation completion did not allow crystal solubilization or dispersion. EDTA had a far more disturbing effect upon the MTT test result. In Mueller-Hinton Broth, 1 hr incubation with micromolar EDTA concentrations resulted in a significant loss of ability of cells to divide on solid media (CFU counts reduced by more than 50%). This viability loss (in the traditional understanding of viability as the ability to form colonies on agar) was not accompanied by an equal MTT test decrease. On the contrary, 0.03 mM EDTA resulted in an increase above the one in untreated control, despite reduced CFU score of the assay mixture (Figure 6). Irreversible injury of P. mirabilis outer membrane by sonication was similarly accompanied by higher MTT reduction activity of the sample. Formazan production was detected even in cell suspensions subjected to 5 min of sonication with maximum power of 600 W (Figure 7). Thus, in gram negative microorganisms, the MTT test result may be unreliable when new compounds of unknown influence on the outer membrane integrity are evaluated as antimicrobials. Moreover, susceptibility assayed by the MTT test may be inconsistent with determinations based on other techniques, leading to confusion in result interpretation. A number of viability definitions were proposed giving the base for different protocols, e.g. standard colony counts demonstrate bacteria maintaining the ability to divide and grow on solid media while MTT assay indicates viability as enzymatic activity of cells. Thus, the MTT assay can be described as a more sensitive method, indicating even the slightest activity alternation, but regrettably more prone to inaccuracies. Nevertheless, as the method of direct viability evaluation, the test is undoubtedly more informative than optical density measurements, which are reliable only if no contamination or dead cells are present. It should be emphasized that the mentioned methods can be characterized as cheap and considerably simple to perform. Fluorolabeling is another approach that can serve for susceptibility testing. Unluckily, it is expensive and often it utilizes hazardous stains. It also usually requires highly purified bacteria cultures and can be significantly sensitive to inorganic contaminations. Although carefully conducted fluorescence staining allows obtaining the most accurate results and unequivocally distinguishes between dead and living cells, MTT test is significantly cheaper, simpler, safer and offers better time-stability than the most popular fluorescence dyes. Under standardized conditions, MTT allows to obtain reliable results respecting not only cell viability but also integrity of its membrane. In our opinion, the microbial MTT test is indeed a convenient real-time indicator of changes in the biochemical status of the cell upon the presence of external agent; however, conclusions concerning their bactericidal effectiveness should be drawn with caution when based solely on this method. It was proposed that the MTT assay methodology for P. mirabilis slightly differs from generally established protocols in a few important aspects. The use of higher bacteria concentration for limited period of time 4,23 allows to remove any inaccuracies arising from bacteria growth. The proper selection of the reaction medium allows to omit the steps in aspiration of the wells 12 after MTT reduction and add the solubilization agent directly to the reaction mixture. Moreover, final formazan dissolution can be performed as originally described 4 or even skipped if Triton X-100 is added to reaction mixture; however, the concentration of detergent must be carefully selected to minimize its antimicrobial 21 or cell permeabilizing action. The protocol is not only simplified but also it highly improves the accuracy of the test by limiting the number of necessary steps.
Conclusion :
The most favorable condition for studying the MTT reduction by P. mirabilis PCM 543 was suspension in PBS containing 0.01% Triton X-100 and 0.5 mg ml-1 MTT using a concentration of 1010 CFU ml-1. The absorbance was read at 550 nm after 30 min incubation at 37°C without incorporation of an organic solvent. Compromising the outer membrane integrity may lead to a false high score of the MTT assay in gram negative microorganisms. This feature of the MTT assay could be advantageous when used as a simple and rapid method for detection of permeabilization or cell envelope disruption in microbiological protocols.
Acknowledgement :
The study was supported by Polish National Science Centre research grant NCN 2011/03/B/NZ6/04964.
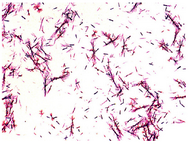
Figure 1. Light microscope observation of Proteus mirabilis PCM 543 culture during the MTT test.
|
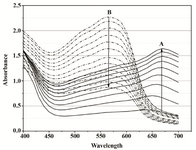
Figure 2A. Overlays of consecutive visible absorbance scans of Proteus mirabilis PCM 543 assay (108 CFU ml-1) in Mueller-Hinton Broth in the presence of 0.5 mg ml-1 MTT. Ascending black lines (A) represent the first 90 min of MTT reduction recorded in 10 min intervals, after that time acidic isopropanol was added; descending dot lines (B) were recorded for 110 min after acidic isopropanol addition.
|
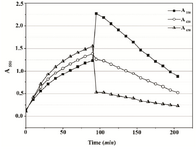
Figure 2B. Absorbance changes in Mueller-Hinton Broth over 90 min of MTT reduction by Proteus mirabilis PCM 543 and subsequent 110 min of formazan solubilization with acidic isopropanol. At 650 nm, cell suspension density was followed, at 620 nm cell-formazan crystals complexes, and solubilized formazan at 550 nm.
|
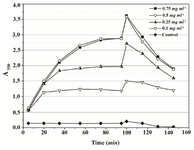
Figure 3. Time-course of changes of absorbance at 550 nm during both steps of Proteus mirabilis PCM 543 MTT assay at various substrate concentrations. Acidic isopropanol was incorporated at 90 min.
|
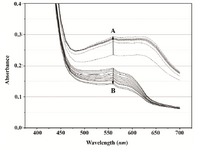
Figure 4A. Overlays of consecutive visible absorbance scans of Proteus mirabilis PCM 543 assay (108 CFU ml-1) in PBS in the presence of 0.5 mg ml-1 MTT. Arrow A shows the direction of absorbance changes during the MTT reduction step, arrow B during formazan solubilization with acidid isopropanol.
|
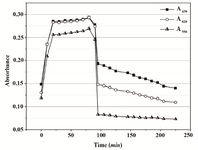
Figure 4B. Absorbance changes in PBS over 90 min of MTT reduction by Proteus mirabilis PCM 543 and subsequent 110 min of formazan solubilization with acidic isopropanol.
|
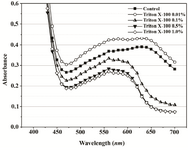
Figure 5A. Visible spectra of Proteus mirabilis PCM 543 assays at various Triton X-100 concentrations. Absorbance was recorded after 60 min of MTT reduction in PBS prior to acidic isopropanol addition.
|
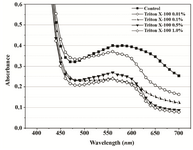
Figure 5B. Visible spectra of Proteus mirabilis PCM 543 recorded 20 min after acidic isopropanol addition and used to terminate 60 min assay in PBS at various Triton X-100 concentrations.
|
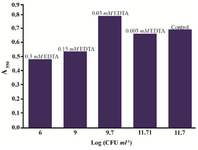
Figure 6. Proteus mirabilis PCM 543 MTT test results in the presence of various EDTA concentrations (values above bars) versus CFU score of reaction mixture samples collected before solvent addition.
|
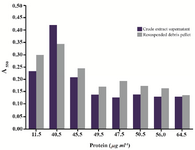
Figure 7. Results of the MTT assay conducted with Proteus mirabilis PCM 543 cells subjected to sonication. Pair of bars represents assays containing either supernatant or resuspended cell debris collected after each disintegration cycle versus protein content of the crude extract.
|
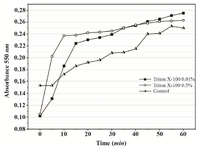
Figure 8. Continuous MTT assay recorded over 60 min in the presence of 0.01% and 0.05% Triton X-100. In the control, time-course of MTT reduction was followed in a set of separate assays terminated with acidic isopropanol at various time points.
|
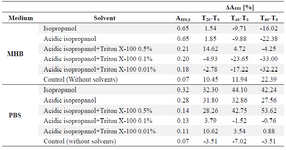
Table 1. Dissolution of commercially obtained formazan crystals in the presence of Triton X-100
|
|