The Effects of Ultraviolet Light and Riboflavin on Inactivation of Viruses and the Quality of Platelet Concentrates at Laboratory Scale
-
Mirshafiee, Hamideh
-
Department of Microbiology, Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran
-
 Sharifi, Zohreh
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tel: +98 21 82052233; E-mail: z.sharifi@ibti.ir
Sharifi, Zohreh
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran, Tel: +98 21 82052233; E-mail: z.sharifi@ibti.ir
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
Hosseini, Seyed Masoud
-
Department of Microbiology, Faculty of Biological Sciences, Shahid Beheshti University, Tehran, Iran
-
Yari, Fatemeh
-
Blood Transfusion Research Center, High Institute for Research and Education in Transfusion Medicine, Tehran, Iran
-
Nikbakht, Hamed
-
Laser and Plasma Research Institute, Shahid Beheshti University, Tehran, Iran
-
Latifi, Hamid
-
Laser and Plasma Research Institute, Shahid Beheshti University, Tehran, Iran
Abstract: Background: This study investigated the effects of Riboflavin (RB) combined with different doses of UV on Platelet Concentrate (PC) which was infected by three models of virus. Platelet quality after treatment was also assessed.
Methods: Three models of virus used in this study were Vesicular Stomatitis Virus (VSV), Herpes Simplex Virus (HSV), and Polio virus, which were added to PC. After photochemical treatment with RB and UV light, residual viral infectivity was titrated using 50% Tissue Culture Infective Dose (TCID50)/ml. This treatment was done with concentration of 50 µM of RB and different doses of UV light (0.24, 0.48, 0.97, 1.29 J/cm2). Platelet quality was assessed by measuring pH, Lactate Dehydrogenase (LDH), MTT assay and cell count after treatments and during 4 days of storage against control groups.
Results: Concentration of 50 µM RB with combination of 1.29 J/cm2 dose of UV resulted in the highest titer reduction of VSV (4 log 10) and HSV (4.26 log10) and lowest titer reduction of Polio virus (2.6 log10). No significant difference was observed between different doses in comparison with control groups. In all treatment groups, the storage stability of platelets in PC was in the acceptable range in comparison with control group.
Conclusion: This study indicated that RB/UV treatment was a promising pathogen reduction technique in PC and had limited effects on platelet quality. However, further optimization of this method is necessary to deal with blood-borne viruses like non-enveloped viruses.
Introduction :
Despite various proactive interventional approaches to blood safety which embody a more stringent donor screening, introduction of new exclusion criteria, registries of previously deferred donors, various specific serological and Nucleic Acid based Testing (NAT), post-donation tracking and transfusion haemovigilance, etc., Transfusion Transmitted Infections (TTI) still could occur 1. These residual risks are due to relatively high incidence of bacterial contamination of platelet (PLT) products. Additionally, pathogen levels below the detection limit of a screening test during acute infection (window period) or the presence of newly emerging agents like West Nile, Dengue or Chikungunya viruses and parasites such as Trypanosoma cruzi, Plasmodium falciparum, or Leishmania continue to raise the potential for disease transmission by blood products 1,2.
Several physical, chemical or photochemical processes have been developed to broadly and non-specifically inactivate or reduce pathogens and increase blood supply safety. The principle of this method is based on light treatment of the respective product in the presence of a photoactive substance (a photosensitizer) which absorbs light of defined wavelengths and transfers the absorbed energy to an electron acceptor to inactivate the pathogen contaminating a blood product 3.
One of the main photochemical processes that have been validated for both platelets and plasma components is the use of Riboflavin (RB) combined with UVB (280-360 nm) light. RB acts by intercalation with nucleic acids and, when exposed to light, causes damage to DNA by producing single oxygen, electron transfer, hydrogen peroxide, and hydroxyl radicals, all of which lead to nucleic acid basic damages and DNA strand breaks. It seems that the combination of RB and UV selectively enhances damage to the guanine bases in DNA. It was suggested that type and extent of damage to DNA for virus in the presence of RB and light make it less likely to be repaired by normal repair pathways available in host cells 4. Platelets are anucleated cells derived from megakaryocytes which typically circulate for 10 days 5. The challenge for all Pathogen-Reduction Technologies (PRTs), however, is to maintain biological function of the treated blood cells, while achieving adequate pathogen reduction levels.
Considering the implementation of this technique into routine use, the question arises whether this pathogen reduction method has an influence on the quality and storability of the platelets. Light of shorter wavelength like UVB (280-315 nm) and UVC (200-280 nm) has detrimental effect on the platelets and proteins through the generation of active oxygen species. Therefore, for establishment of new technology in the country, it is necessary to perform studies in order to achieve sufficient benefit for implementation of pathogen inactivation.
In this study, the ability of UV light with the radiation peak of 365 nm, RB and RB accompanied with UV light treatments of Platelet Concentrate (PC), as a physical means to inactivate model viruses and also the influence of this procedure on the in vitro quality of platelets in comparison to control were investigated.
Materials and Methods :
Day (d) 0 was defined as the day of collecting platelets and the day of treatment of PC was named d1. In this study, three control groups were used to investigate the effects of UV light and RB alone on virus and PC quality.
In control group 1, PC was treated with different doses of UV light, without RB. The light intensity was 0.00203 W/cm2. The different doses of UV light were 0.24, 0.48, 0.97, and 1.29 J/cm2, and exposure times for these doses of UV light were 2, 4, 8 and 10 min, respectively. This control group has been defined by CUV-PC. In control group 2, PC was treated with RB. No UV treatment was accomplished. This control group has been defined by CRB50µM-PC and CRB25µM-PC. In control group 3, PC was used without UV light and RB. This control group has been defined by C-PC.
In the first stage, treatment with RB+UV light was used for inactivation of only one model virus (Vesicular Stomatitis Virus: VSV). In this stage, effects of UV (CUV-PC) and two concentrations of RB (CRB50µM-PC and CRB25µM-PC) were independently investigated on VSV inactivation compared with C-PC.
In the second stage, the efficacies of UV light and 50 µM concentration of RB were examined for inactivation of two viruses; Polio virus and Herpes Simplex Virus (HSV).
In the third stage, the influence of UV light and 50 µM concentration of RB was investigated on the in vitro quality of platelets in comparison with control groups, during 2 and 4 days of storage. In this study, platelet metabolism, activation and mitochondrial function parameters during the storage were the main focus of the study.
Preparation of platelet concentrates: PCs were obtained from the Iranian Blood Transfusion Organization (IBTO). PCs were prepared by the Platelet-Rich Plasma (PRP) method according to a standard protocol 6 in Iranian Blood Transfusion Organization. In brief, the whole blood (350 ml) was collected in double blood bags (JMS Singapore, Ltd.) including Citrate Phosphate Dextrose Adenine (CPDA-1) solution as anticoagulant. After a resting time of 30 min, the whole blood was centrifuged by soft spin centrifuging at 22°C to prepare PRP followed by a high speed centrifugation to obtain a platelet pellet. Most of the plasma was removed, and the platelets were stored in reduced volume of remaining plasma 6,7.
Riboflavin preparation: Five hundred µM of RB was dissolved in a 0.9% sodium chloride solution with pH=4-5 8. It was sterilized by steam autoclave process. The solution was overwrapped in an opaque foil pouch to protect it from the light. Then, it was used freshly for each experiment. Two concentrations of 25 and 50 µm were used. For making these concentrations, the stock solution of RB was diluted in PC to obtain the final concentration of 25 or 50 µM.
Virus preparation: VSV and HSV were grown and titrated in Vero cell line (NCBI Code: C101, Pasture Institute of Iran). Polio virus was propagated and titrated in HeLa cell line (NCBI Code: C115, Pasture Institute of Iran) 9.
Small-scale illumination: A mixture of virus (1 ml) and RB (25 or 50 µM) was added to the volume of 10±0.5 ml of PC and then illuminated in a petri dish (9 mm in diameter). This resulted in a fluid layer with a thickness of approximately 2 mm. The illumination device with a 200-W mercury-xenon lamp (Hamamatsu Lightning Cure LC5) emitted UV light at the wavelength of 200- 365 nm. The energy density was 0.00203 W/cm2. Different doses of UV (0.24, 0.48, 0.97 and 1.29 J/cm2) light were used in this study.
Virus inactivation studies: Virus suspension and RB (25 or 50 µM) were spiked 1:10 with PCs (volume, approx. 10 ml; n=3 per test for each virus) and then were irradiated (see above). After treatment with RB and different doses of UV light, viral titers were determined by endpoint titration in 96-well microtiter plate assays using 4 replicates for each sample. The plates were incubated at 37°C in humidified atmosphere containing 5% CO2. After an appropriate incubation period, the cell layers were inspected microscopically for the cytopathic effects (CPE). Titers were calculated using the method of Reed and Muench and were expressed as log of tissue culture infectious doses (TCID50) 9.
In brief, the reduction factors were calculated by the following equation: R= Log 10 A0- Log 10 At, where R is the reduction factor, A0 is the total virus load after spiking, and At is the total virus load in the treated sample.
Sampling and cell quality analysis: To investigate the quality of treated PCs, they were transferred to a storage bag (BEASAT Ind. Co., Iran) using syringe 16G, and stored for 4 days, under standard blood bank conditions, at 22ºC under gentle agitation. The samples were drawn aseptically for cell quality analysis on days 2 and 4. The samples were assayed for platelet count (Sysmex, K-1000, Kobe, Japan), pH, LDH (Pars Azmon Co., IRAN) and MTT reduction assay (Roche, Germany).
Platelet enumeration: The number of platelets was determined using an automated electronic particle counter (Sysmex, K-1000, and Kobe, Japan). For this purpose, 0.1 ml of PC was added to 0.4 ml of phosphate buffer saline (PBS).
Measurement of lactate dehydrogenase activity: LDH activity was measured by colorimetric methods using assay kit (Pars Azmon Co., Iran). LDH activity was measured at 340 nm.
Analysis of the cell viability using MTT assay: In order to measure the metabolic activity of platelet concentrates, the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT, Roche,Germany) was used. The MTT assay is a colorimetric method to assess cell proliferation and viability. NADPH-dependent mitochondrial oxido-reductase enzymes are capable of reducing the tetrazolium due to insoluble formazan. This enzyme activity is dependent on the survival condition of the cell. The cell viability rate was directly correlated with the amount of purple formazan crystals 10. This metabolic redox activity of PC was measured to assess platelet viability during platelet storage. For this purpose, PC was diluted in PBS to a concentration of 3×105 platelets/µl. Then, 100 µl of diluted platelets were incubated with 10 µl of MTT (5 mg/ml) for 4 hr at 37ºC. After addition of 100 µl of lysis buffer (10% SDS, 0.02 mol/l HCL) and incubation for one night at 37ºC, the absorbance was measured at a wavelength of 575 nm by the spectrophotometer.
Statistical analysis: Each experiment was done three times, and the average and SD were presented for each sample. Statistical analysis (general linear model analysis of variance [ANOVA]) was carried out using GraphPad Prism® (Version 5.0) software for each experimental condition. According to the standard statistical procedures, a p<0.05 was considered to indicate a statistically significant difference.
Results :
Inactivation of VSV in PCs: In the primary study, the efficacy of UV for VSV inactivation as an enveloped RNA virus was tested by two concentrations of RB (25 and 50 µM). For both concentrations of RB, viral reduction factor was observed at four doses. As shown in figure 1, a dose of 1.29 J/cm2 of UV light for 10 min was able to reduce VSV titer about 3.8, 4.06 and 3.01 log10 for the concentrations of 25 µM, 50 µM of RB and CUV-PC, respectively. Also, in 50 µM concentration of RB, 1.16 logs 10 of VSV was inactivated by 0.24 J/cm2 for 2 min, but with the same dose, only 0.9 log10 was inactivated for 25 µM and 0.6 log10 for CUV-PC. No Statistically significant difference was found between the two concentrations of RB. CRB-PC was used for the evaluation of the effects of RB alone in the process of virus inactivation and none of RB concentrations were critical factors for virus inactivation (data not shown).
Inactivation of polio virus and HSV in PLTs: The optimum concentration of RB was employed to inactivate HSV and Polio virus. Table 1 shows the effect of RB (50 µM) accompanied by different doses of UV for the inactivation of these viruses.
In vitro PLT quality after treatment and storage: Treated PCs were tested for pH, LDH activity, mitochondrial enzymatic activity (MTT assay), and cell count after treatment during storage on days 2 and 4 at 22oC. The results were compared to control groups. Control groups were collected, processed, and stored in the same condition. In this research, the effect of concentration of RB and UV on the quality of platelets was studied. The results of the quality analysis of PCs after treatment during storage on days 2 and 4 are summarized in tables 2 and 3. As shown in table 2, CRB-PC and CUV-PC show that RB or UV alone did not have any significant effect on platelet quality. Also, in parallel experiments, no significant difference was observed between the control and other treated units on days 2 and 4. Direct correlation was observed between quality of platelet and UV dose rate. Increasing doses of UV resulted in clear deterioration in cell count, mitochondrial enzymatic activity (MTT), and LDH. These changes were not statistically significant.
Cell count enumeration during storage of PC after treatments: In parallel with the increasing dose of UV, platelet count declined in all samples, but no significant difference was obtained between the control (C-PC) and treatment groups after treatment and during storage (Tables 2 and 3).
pH changes during storage of PC after treatments: There was no statistically significant difference between the C-PC and treatment groups (Table 2). pH values for all of the samples remained above 7.0 during 4 days of storage (Table 3).
Changes in LDH during storage of PC after treatments: As shown in table 2B, increasing doses of UV resulted in clear deterioration of LDH, but there was no significant difference in LDH level between C-PC and treatment groups (Table 2). LDH levels were raised in all samples during storage (Table 3).
MTT assay during storage of PC after treatments: MTT assay was used for detecting living/dead cells (viability assay). As shown in tables 2A and 2B, different doses of UV resulted in reduction in platelet survival, but the difference was not significant. The greatest decrease in cell survival was observed by RB in combination with UV 0.24 J/cm2 (this dose was used for 2 min) which was not statistically significant (Table 2B). Cell survival decreased in all treatment groups and control groups during storage, but no statistically significant difference was observed between the C-PC and treatment groups during storage (Table 3).
Discussion :
Platelet transfusion is a mainstream therapy for preventing or treating bleeding episodes in thrombocytopenic patients or patients with a high risk of bleeding 11. The role of platelets in hemostasis is to migrate to sites of injury or damage, bind to the damaged surface, and release agents that stimulate tissue repair and recruitment of other platelets to the site of injury. Success in platelet transfusion depends on the cellular viability and homeostatic activity of the transfused platelets and on the physiological status of the recipient 12.
Inactivation of pathogens using photochemical or dynamic procedures is a promising technique to improve blood safety of cellular blood products. These processes may cause damage to the blood components, thereby resulting in the shortening of the lifespan of platelets during storage 13. For evaluating the success of a PRT for PCs, there are four main factors that must be taken into account. They are ease of use, cell quality, pathogen reduction levels, and toxicity.
Studies show that 50 µM concentration of RB in combination with UV light at 6.2 J/cm2 with an output ranging from 265 to 370 nm can inactivate pathogens (like Porcine parvovirus, cell associated and cell free HIV, WNV, plasmodium spp., Trypanosoma cruzi, Escherichia coli, Staphylococcus epidermidis) in plasma and PC 8,14.
The results of this study showed that concentration of 50 µM of RB combined with dose of 1.29 J/cm2 (exposure time for this dose was 10 min) in comparison with CUV-PC and the concentrations of 25 µM and 50 µM of RB with combination of different doses of UV, was sufficient to inactivate VSV titer at least about 4.06 log (Figure 1). Our data indicated that with increasing the UV light dose, all parameters tested underwent changes until day 4, but these changes were not significantly different between C-PC and the treatment groups. Apparently, PC damage by RB plus UV light was limited under the condition used (Tables 2 and 3). According to the American Association of Blood Banks (AABB), the pH level of ≥6.2 is an essential requirement for quality control of blood components 15. The results of this study showed that increasing exposure of light had no significant effect on the average of pH levels in the PCs tested and pH levels had the accepted range during storage.
Platelet membrane integrity can also be assessed by measuring the amount of the LDH enzyme that leaks from the cell. Although LDH level was raised during the storage in all of the samples, LDH levels were slightly lower in the treated PC with increasing illumination in comparison with the control, except dose 0.24 J/cm2 (equal to 2 min). It seems that rate of used doses (0.48, 0.97, 1.29 J/cm2) does not harm the integrity of platelet membrane.
Given the fact that MTT is related to mitochondria-based dehydrogenase activity 16, our observation showed that compared to the control, treatment of PCs with RB did not significantly affect their survival and the mitochondrial function (except for dose 0.24 J/cm2). MTT for controls and treated PCs did not significantly change during storage for up to 4 days. Our results on treated PC for MTT assay is also consistent with observations by Li et al (they had used RB (50 μM) plus 5 J/cm2 UV light) 17 and they showed that cell viability was lower in the test than that of control on day 4 of storage (Table 3).
This study showed no significant change in cell count after treatment. Post-treatment storage for 4 days did not induce a significant decrease in cell number for either treated PCs. Several studies have been conducted to assess the impact of RB and UV light on PC quality 18,19. Previous studies which were designed to use RB at a final concentration of approximately 50 μmol/l and UVB light from a fluorescent lamp with the energy of 6.24 J/ml, have not shown remarkable change in the parameters of pH, LDH, MTT assay and cell count during storage 20,21.
It seems worthy to note that UV dose of 0.24 J/cm2 for 2 min, compared to other doses further reduced the viability of cells and had increased LDH level. With regard to cell, damage with this dose was higher than others; therefore, it seems that the time of illumination influenced the platelet quality.
No significant differences in log reduction of VSV titer were observed between different concentrations of RBs. So, pathogen inactivation was carried out with the concentration of 50 μM of RB. Although with raising the dose of illumination, the enveloped viruses, HSV, and VSV (up to 4.26 and 4.06 log10, respectively), were effectively inactivated at 1.29 J/cm2, logs reduction of virus titer for Polio virus that is a non-enveloped virus, was 2.6. This result indicated that the enveloped viruses were more sensitive than non-enveloped viruses. Consistently, other studies have shown inactivation of enveloped viruses, such as West Nile Virus (WNV), HIV, VSV, and influenza A was more effective than non-enveloped viruses, such as human hepatitis A and encephalomyocarditis virus 22.
This study suffered from some limitations such as unavailability of irradiation bags and the absence of agitation during illumination, which could contribute to reduction of the exposure time.
Conclusion :
In summary, there was no significant variation in the values of studied parameters, which, therefore, indicated that the treated PCs with different doses of UV were generally still within acceptable limiting range at the end of the four day storage period. Also, this report showed that the UV dose of 1.29 J/cm2 was effective in inactivating VSV and HSV which were representative of enveloped RNA viruses and enveloped DNA viruses, respectively.
Acknowledgement :
This study has been fulfilled with the financial support of the High Institute for Research and Education in Transfusion Medicine, Blood Transfusion Research Center. We gratefully acknowledge the advice and technical support of the staff of virology laboratory of the Iranian Blood Transfusion Organization.
Conflict of Interest :
None of the authors have any conflicts of interest to declare.
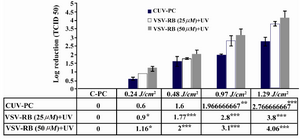
Figure 1. The effect of UV light treatment on VSV inactivation using 25 µM, 50 µM of RB and Cuv-PC. As it could be seen in this figure, titer of VSV had the highest reduction by RB (50 μM) combined with different doses of UV light.
* p<0.05, p<0.001, *** p<0.0001
|
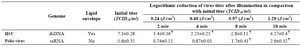
Table 1. Effects of RB (50 µM) in combination with different doses of UV light on HSV and Polio virus (The data represent mean±SD)
¶ P<0.0001
|
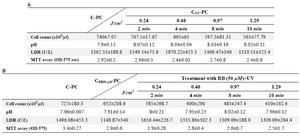
Table 2. Evaluated parameters of cell quality on day 2 (The data represented mean±SD). Effects of UV alone in different doses on platelet quality (1A). The effects of RB (50 µM) alone and RB in combination with UV light on the platelet quality (1B)
|
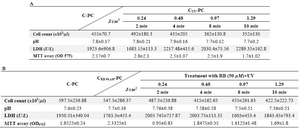
Table 3. Evaluated parameters of cell quality on day 4 (The data represented mean±SD). Effects of UV light alone in different doses on platelet quality (3A). The effects of RB (50 µM) alone and RB in combination with UV light on the platelet quality (3B)
|
|