ADSCs on PLLA/PCL Hybrid Nanoscaffold and Gelatin Modification: Cytocompatibility and Mechanical Properties
-
 Mashhadikhan , Maedeh
Department of Biology, Faculty of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran, Tel: +98 183641974, Email:m.mashhadikhan@srbiau.ac.ir
Mashhadikhan , Maedeh
Department of Biology, Faculty of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran, Tel: +98 183641974, Email:m.mashhadikhan@srbiau.ac.ir
-
Department of Biology, Faculty of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Soleimani, Masoud
-
Department of Hematology and Blood Banking, Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran
-
Parivar , Kazem
-
Department of Biology, Faculty of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran
-
Yaghmaei, Marjan
-
Department of Biology, Faculty of Sciences, Science and Research Branch, Islamic Azad University, Tehran, Iran
Abstract: Background: Development of tissue engineering and regenerative medicine has led to designing scaffolds and their modification to provide a better microenvironment which mimics the natural niche of the cells. Gelatin surface modification was applied to improve scaffold flexibility and cytocompatibility.
Methods: PLLA/PCL aligned fibrous scaffold was fabricated using electrospinning method. ADSCs were seeded after O2 plasma treatment and gelatin coating of the scaffolds. The morphological and mechanical properties of blends were assessed by Scanning Electron Microscopy (SEM), tensile test and ATR-FTIR. The cells proliferation was evaluated by MTT assay.
Results: Based on the results, it is supposed that gelatin coating is a brilliant method of surface modification which significantly increases the mechanical properties of scaffold without any changes on the construction or on the direction of nanofibers which conducts cell’s elongation. MTT analysis exhibited that ADSCs attachment, viability and proliferation significantly (p<0.05) increased after gelatin treatment.
Conclusion: Gelatin surface modification is a highly beneficial method to improve cytocompatibility, flexibility and mechanical features of the scaffolds which doesn’t affect the nanofibers construction. Proliferation of Adipose Derived Stem Cells (ADSCs) as a remarkable source of stem cells was investigated for the first time on PLLA/PCL hybrid scaffold.
Introduction :
Tissue engineering has opened a widespread context of studies on using three dimensional (3D) cell cultures via imitating the extracellular matrix (ECM) topography to design the scaffolds 1-3. Nanofibrous scaffolds have porous structure with large surface area which mimic the natural microenvironment of ECM; the blends regulate the cell attachment, nutrition, proliferation and differentiation 4,5. Among the material choices to fabricate scaffolds, Poly (l-lactide) acid (PLLA) and Poly (ε-caprolactone) (PCL) due to their numerous advantages of biodegradability, easy access and application, mechanical properties and biocompatibility, have been used extensively 6,7. PLLA, a polyhydroxy acid, is synthesized from poly l-lactic acid which is derived from fermentation of corn, sugar beet or potato. This non-toxic polymer is approved by US Food and Drug Administration (FDA). It is relatively stiff and brittle (semi-crystalline) and its degradation and absorption is in aqueous medium. Several studies showed that PLLA has a long term stability of 24 months in vivo 2,8-10, the same time that injured human tissue needs to be cured 11. The PCL, another aliphatic, semi-crystalline and non-toxic polyester is utilized for its rubbery state that could enhance the flexibility of the scaffold. However, PCL is highly hydrophobic which results in poor cell attachment in vitro, therefore, it needs to be modified before cell seeding through plasma treatment, or by coating the scaffold or blending with other materials 2,6,10,12. PLLA/PCL hybrid is well investigated because of its favorable qualities such as degradation rate, porosity and resistance to high temperature and pressure; PLLA 1,8,13, PCL 3,14,15 and their blends 2,6,7,9,16-20 were well investigated in the literature due to many of their physicochemical, morphological, thermal and mechanical properties.
Among various methods of making PLLA/PCL hybrid, electrospinning is an optimal system to fabricate different types of nanofibrous scaffolds from a variety of materials 2,9,10,16,18. Accordingly, researches on fibrous scaffolds authenticate the role of fiber orientation in controlling the cell’s elongation 10,21-23. Surface coating is a modification method utilized to improve mechanical structure, hydrophilicity, bioactivity and cytocompatiblity of the scaffolds by use of different biomaterials 11,24,25. Because of high hydrophobicity of PLLA/PCL blends and poor cell attachment, O2 plasma treatment was applied for all samples in this study 11,24. Moreover, blends were coated with gelatin which has recently been applied in tissue engineering as a non-antigenic biomaterial.
Controlling of nanofiber formation is very important in electrospinning and the plasticity and stability of hybrids depend on choosing the correct biomaterials. Hence, despite various studies on gelatin direct blending with the scaffolds 3,14,26, it is preferred to coat the blends with gelatin after electrospinning 11. Gelatin is made of disintegration or thermal denaturation of collagen. It provides better cell attachment and proliferation of the scaffolds; likewise, gelatin coating is a simple way to increase the mechanical competence of the scaffolds without affecting the interconnectivity of the pores or reducing cytocompatibility of the scaffold 27,28. Regarding gelatin benefits of no effect on scaffold bioactivity 27, non-antigenicity 28 and simplicity of use, it was applied to modify the surface of the blends. Several studies on surface modification of PLLA through gelatin coating declared that the hydrophilicity, mechanical property and cell attachment and differentiation of the scaffold, were considerably augmented after these treatments 11,13,25,29-31.
The goal of tissue engineering is searching among different biomaterials to find the suitable one for fabricating scaffolds and finding various methods of scaffold modifications, in order to be utilized in cell culture issues; in other words, the response of cells in case of applying these materials should be investigated 24,32,33. Mesenchymal Stem Cells (MSCs) are multipotent progenitor cells which have been isolated from various adult tissues. Among them, Adipose Derived Stem Cells (ADSCs) are favorable sources of stem cells which express all typical surface markers and have all MSCs capabilities and characteristics, but also they have highest proliferation rate and apoptosis tolerance. The most important point is, ADSCs can be obtained from very small amount of tissue whereas large amount of it can easily be obtained under local anesthesia which is non-invasive and doesn’t result in awful injury or pain 32,34,35. In addition, the effect of aging and multiple passages on proliferation and differentiation ability of ADSCs is less than that of BMSCs 33. These advantages encouraged us to investigate the growth and elongation of ADSCs on aligned PLLA/PCL hybrid which is coated or not coated with gelatin.
In the present study, the use of gelatin coating was investigated as a novel way of surface modification which significantly promotes the mechanical properties and cytocompatibility of PLLA/PCL hybrid scaffold and also it has no effects on scaffold construction.
Materials and Methods :
Scaffold fabrication: Poly (l-lactide) acid with molecular weight of 240,000 g/mol (Sigma-Aldrich, MO, USA) and poly (ε-caprolactone) with MW of 80,000 g/mol (Sigma-Aldrich, MO, USA) were dissolved in chloroform (Merck, Germany) and dimethylformamide (DMF) (Sigma, Aldrich), in order to synthesize PLLA/PCL blend via electrospinning method. PLLA was first dissolved in chloroform and then DMF (4.25:0.75), while PCL dissolved in choloroform/DMF (8:2). Next, they were separately stirred for 3 hr. Polymer solutions were loaded into 5 ml plastic syringes that each one was connected to a 21-gauge needle. By applying the positive voltage between needles and collector (18 kV for PLLA and 24 kV for PCL), solution droplets left the needles to form nanofibers while deposited on collector. The percentage of PLLA/PCL was 47/53 wt%. The needle tips were placed at a distance of 15 cm from collector for PLLA and 20 cm for PCL, while rotating disk with the linear rate set to 2800 rpm to collect aligned nanofibers. Surface modification included oxygen plasma treatment performed by a low frequency plasma generator (Diener Electronics, Germany) for 5 min and gelatin coating. Due to PLLA/PCL high hydrophobicity, it exhibited very low cell attachment without plasma treatment, therefore, plasma treated hybrids were used in all experiments of this survey.
The hydrophilicity testing: The water contact angle of the surface of scaffolds was measured at room temperature by sessile drop method with a G10 contact angle goniometer (Kruss, Germany). The contact angle was measured after 10 s of placing a drop of deionized water on the blends before and after plasma treatments and coating with gelatin.
ATR-FTIR spectroscopy: The surface gelatin coating of PLLA/PCL hybrid was investigated by Fourier Transform Infrared Spectroscopy (FTIR). IR spectra were obtained using Vertex 80 spectrometer (Bruker Optics, Germany) equipped with a DTGS detector and a diamond ATR crystal.
Mechanical characterization: The mechanical features of gelatin coated scaffolds were compared with the uncoated, by the use of a tensile tester (SANTAM Stress machine, Iran). Scaffold samples contained aligned PLLA/PCL blends with and without gelatin coating and they were cut in 60×10 mm2 dimensions through scaffold’s alignment for testing with the speed rate of 50 mm/min. Stress-strain curves were the layout of the results.
Cell seeding on the scaffold: To obtain the best cell attachment and physical characteristics, scaffolds were coated with gelatin (Sigma, Aldrich) and then sterilized. Porcine gelatin powder was solved (1 mg/ml) in 1% acid acetic on stirrer overnight at RT, then the blends were put on this solution at 4°C overnight. ADSCs were isolated from adipose tissue of BALB/c mice obtained from Stem Cell Technology Research Center (Tehran, Iran) and after second passage were seeded on scaffolds (with or without gelatin coating) with the density of 1×104 per well (24 well plates) in Dulbecco’s Modified Eagle’s Medium (DMEM, Invitrogen, USA) supplemented with 10% Fetal Bovine Serum (FBS; Invitrogen, USA), penicillin (100 U/ml, Gibco) and streptomycin (0.1 U/ml, Gibco), incubated at 37°C with 5% CO2.
Scanning electron microscopy (SEM): After 1 day of cell seeding, the scaffolds were washed twice with PBS, and then fixed with 2.5% glutaraldehyde at 4°C for 2 hr. After dehydration by gradient of alcohol, the scaffolds were kept overnight under fume hood for air-drying, then gold sputtered in vacuum and imaged by EM-3200 digital scanning electron microscope (KYKY, China).
Evaluation of cell viability and proliferation on the scaffold: The cultured ADSCs on the surface modified scaffolds were stained by 0.5 µg/ml of fluorescent DNA-binding dye, 4-6-diamidino-2-phenylindole (DAPI), for 5 min to confirm their existence on PLLA/PCL scaffold. In addition, colorimetric assay was applied to measure the reduction of yellow 3-(4,5-dimethythiazol-2-yl) 2,5diphenyl tetrazolium bromide (MTT) by mitochondrial succinate dehydrogenase, to compare the ADSCs viability and proliferation on scaffolds coated or not with gelatin. MTT assay was performed during 5 days of culture by using an Eppendorf Bio-Photometer (Germany) to read the cell’s OD at 570 nm.
Statistics: Quantitative results were evaluated and statistical analysis was carried out using analysis of variance (ANOVA). Data obtained from triplicate samples (n=3) were expressed as mean standard deviation (SD) and were considered statistically significant when p<0.05.
Results :
Morphological structures: The SEM images illustrate the electrospun PLLA/PCL hybrid, without (Figure 1A) and with (Figure 1B) gelatin coating. The observation of highly porous fiber structure of scaffolds shows that when gelatin was used to cover the blends, obviously the brut and fragile fibers of blend became more supple and flexible, which shows the elastic construction of ECM. It is perceived that gelatin uniformly softened the roughness and dryness of fibers when it was used to coat the scaffolds. However, gelatin solution perfectly covered the nanofibers though it did not blemish or fill the scaffold’s pores distinctly. SEM images also demonstrated that when ADSCs are seeded on scaffolds with parallel pattern, the fibers can conduct the cell’s elongation through themselves. The point is that it happens even after just 1 day of cell seeding (Figures 1C and D) and it indicates the powerful effect of nanofiber orientation as an ECM-like structure on cell growth guidance. Comparing ADSCs on gelatin coated (Figure 1F) and gelatin uncoated (Figure 1E) scaffolds, it can be inferred that in both situations, cells get parallel alignment along the axes of the fibers, thus gelatin coating does not impress cell guidance of the nanofibers. To confirm the presence of the cells on gelatin coated PLLA/PCL scaffold, the cells were stained by DAPI (Figure 2).
Hydrophilicity of blends: Because PLLA/PCL hybrids before plasma treatment were highly hydrophobic (135° angle), it was necessary to apply O2 plasma treatment before any surface modification 24. Contact angle measurements after plasma treatment present that PLLA/PCL hybrid is completely hydrophilic (0° angle) and gelatin coating has no influence on its hydrophilicity.
ATR-FTIR: ATR- FTIR was performed to confirm the existence of gelatin on the surface of PLLA/PCL blends (Figure 3). The peak at 2946 cm-1 corresponded to C-H stretch while at 1735 cm-1 it was for C=O bond and the C-O bending peak appeared at 1182 cm-1. However, the most characteristic peaks of PLLA/PCL were in vicinity of Amide I (1640 cm-1), Amide II (1540 cm-1) and even Amide B (2930 cm-1) of gelatin and the gelatin concentration was so diluted, but the results displayed that a new peak appeared at 1237 cm-1 which confirmed the features of gelatin for Amide III (C-N stretch plus N-H in phase bonding). The asterisks indicate the characteristic peaks of PLLA/PCL in both spectra as if no significant change occurs by gelatin coating.
Tensile properties: Figure 4 illustrates tensile stress-strain curves of PLLA/PCL blends. The non-linear stress-elongation at break curves exhibit an increase in peak of stress when the samples are coated with gelatin in comparison with uncoated ones and this suggests that gelatin increases mechanical properties of the blends. However, data analysis indicates this increase is not remarkable for peak stress (p=0.26) and/or break strain (p=0.63).
Indeed, gelatin modulates tensile properties without damage to mechanical strength of the hybrid.
Cell proliferation: ADSCs showed significantly (p<0.05) higher proliferation rate and viability when they were seeded on PLLA/PCL hybrid in comparison to 24-well Tissue Culture Polystyrene (TCPS) after day fourth, even when the scaffolds were not coated with gelatin. The results of MTT assay suggest that structure of nanofibrous scaffolds improved the cell’s viability. Moreover, MTT results indicated that cell proliferation and viability were augmented when gelatin coating was applied, as it demonstrates significant (p<0.05) increase from day 3 in comparison with both others. It means that gelatin provides a suitable substrate for cell attachment and proliferation compared to uncoated scaffolds (Figure 5).
Discussion :
Electrospun scaffolds have porous structures with large surface area that are the mimic structures of the natural microenvironment of ECM; the nanofiber blends regulate the cell nutrition, proliferation, attachment and differentiation 4,5. Researches on fiber orientations also authenticate the role of scaffolds on controlling the cell elongations 10,21-23.
Surface coating is one of the modification methods utilized to improve mechanical structure, hydrophilicity, bioactivity and cytocompatiblity of the scaffolds by use of different biomaterials 11,24,25. Fortunately, PLLA 1,8,13, PCL 3,14,15 and their blends 2,6,7,9,16-20 were well investigated in literature due to many of their physicochemical, morphological, thermal and mechanical properties. Several studies on surface modification of PLLA through gelatin coating method, declared that the hydrophilicity, mechanical property and cell attachments and differentiations of the scaffold were considerably augmented after these treatments 11,13,25,29-31. Gelatin benefits no effect on scaffold bioactivity 27, non-antigenicity 28 and its simplicity of use encouraged us to modify the surface of blends by gelatin coating.
As it is exhibited in SEM pictures (Figure 1), the watery gelatin solution perfectly covered the nanofibers of the scaffold without any morphological changes in fiber diameters, shape and size of the pores, or scaffold alignments.
The goals of tissue engineering efforts, using different biomaterials to fabricate scaffolds and their modifications, are to utilize these scaffolds for cell culture; in other words, response of cells in case of applying these materials should be investigated 24,32,33. Therefore, ADSCs were used as novel sources of cells to seed on hybrids 32,33. First, the morphological properties of ADSCs on aligned blends were observed based on the surveys in case of other materials and cells 36,37 and our SEM observation also illustrated cell’s elongation which was in line with PLLA/PCL fibers. Furthermore, the gelatin coated hybrids were compared with non-coated ones. SEM pictures present that surface gelatin modification did not bend topographic guidance of the aliged fibers which indicates that gelatin coating maintains the scaffold bioactivity (Figure 1). The cells nuclei are DAPI stained to confirm cell sitting on gelatin coated scaffold (Figure 2). Likewise, the measurements of contact angel show that gelatin did not change scaffold hydrophilicity at all. The gelatin source and concentration should be noticed since high concentration of it may vary the scaffold properties 14,38. The 1% diluted concentration of gelatin which was used to cover the scaffolds had low concentration to alter the scaffold characteristics; the ATR-FTIR spectra confirmed that gelatin finely coated the scaffold unless there were any shifts in PLLA/PCL transition peaks (Figure 3) considering the vicinity of gelatin polypeptide secondary structure peaks with the PLLA/PCL peaks 26,39.
Gelatin surface modification increases flexibility, plasticity and inflection of fibers 14,27; The results from tensile test confirm that gelatin coated samples obviously had higher peak in stress and there was no significant (p>0.05) increase at stress peak or elongation at breaks (Figure 4) which affirms the morphological outcomes. This suggests that gelatin improved mechanical properties of scaffold without making shift or transformation in its construction 13.
Studies on scaffold cytocompatibility demonstrate significant increases of cell proliferation and viability 14,24. Accordingly, in this study, the effects of gelatin coating of PLLA/PCL blend on ADSCs proliferation were investigated; MTT assay was applied before and after gelatin coating on PLLA/PCL blends (Figure 5). MTT analysis displayed significant (p<0.05) increase of ADSC proliferation and viability in case of PLLA/PCL blends after 4 days of culture in comparison with TCPS which presented that PLLA/PCL blends provided better cell attachment, nutrition and microenvironment 36. The point is, this increase is significant (p<0.05) from day 3 in gelatin coated scaffolds versus both uncoated and TCP. Thus, gelatin coating is a beneficial surface modification which increases cell viability, proliferation and attachment of PLLA/PCL blends via improvement of mechanical properties of the nanofibers without affecting hydrophilicity, bioactivity or morphological structure of scaffolds.
In this study, an attempt was made to find a truly simple method of surface modification on aligned PLLA/PCL blend which can improve the mechanical features, plasticity and cell supports. This simple way of gelatin surface modification can elevate cytocompatibility and cell attachments of other kinds of scaffolds which are not biodegradable (e.g. PES) and have low cell attachments. Our results specifically can be used in special conditions of cell culture such as myoblasts of skeletal muscle 37, cartilage 21, neuron 23 and bone 36 differentiation which need mechanical support and alignment of the biodegradable scaffolds, regarding the use of potent ADSCs as a powerful source of stem cells for tissue generation.
Conclusion :
In order to improve the mechanical supports and bioactivity of the electrospun aligned PLLA/PCL scaffolds, gelatin surface coating was used as an extremely effective modification. In spite of low gelatin concentration used for covering the blends, ATR-FTIR confirmed that it perfectly covered the nanofibers of the hybrid. Contact angel measurements showed that gelatin does not change the scaffold hydrophilicity. Moreover, tensile stress-strain curves confirmed the increase of plasticity and mechanical properties of the scaffold while making no significant changes in hybrid construction. SEM images illustrated gelatin surface modification did not affect pore interconnectivity or alignment of the scaffold. To investigate whether or not gelatin affects scaffold alignment, SEM technique was applied and images of cultured ADSCs on scaffolds presented that this treatment has no effects on cell growth in parallel with fibers elongation. Our study on ADSCs during 5 days of culture confirmed that proliferation, attachment and viability of the cells significantly increased when gelatin coating was applied compared with uncoated scaffolds. Consequently, gelatin coating is an ideal method for surface modification of nanofiber aligned PLLA/PCL scaffolds to improve their plasticity in addition to cell proliferation, attachment and viability.
Acknowledgement :
The authors would like to thank Dr. Abdolreza Ardeshirylajimi and Dr. Iman Shabani for their kind assistance.

Figure 1. PLLA/PCL hybrid nanofibers, A) without gelatin coating; B) with gelatin coating; C, D) show ADSCs cultured on aligned PLLA/PCL; the orientation of cells is parallel to the scaffold fiber even after just 1 day of cell seeding. E) ADSCs on aligned PLLA/ PCL without gelatin coating; F) ADSCs on aligned PLLA/PCL with gelatin coating. Scale bars are 100 µm in main pictures and 10 µm in the small boxes (A, B), 1 mm (C) and 100 µm (D, E, F).
|
Figure 2. DAPI staining of ADSCs on gelatin coated PLLA/PCL hybrid scaffold (10x).
|
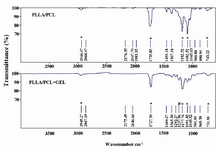
Figure 3. ATR-FTIR spectra of PLLA/PCL and gelatin coated PLLA/PCL. The * indicates characteristic peaks of PLLA/PCL and # indicates peak for Amide III of gelatin.
|
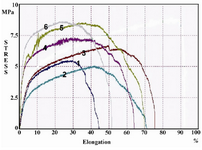
Figure 4. Stress-strain of PLLA/PCL compared with gelatin coated samples. The samples numbered 1-3 are related to uncoated and 4-6 to gelatin-coated hybrids.
|
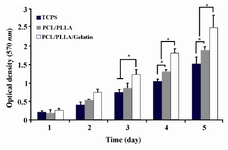
Figure 5. MTT assay of ADSCs proliferation and viability on PLLA/PCL scaffolds with or without gelatin coating and TCPS during 5 days of culture. Significant increase in cell’s OD levels is indicated with * at <0.05.
|
|