Intravenous Transplantation of Very Small Embryonic Like Stem Cells in Treatment of Diabetes Mellitus
-
 Ragerdi Kashani, Iraj
Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 9127019141, Email: Ragerdi@razi.tums.ac.ir
Ragerdi Kashani, Iraj
Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran, Tel: +98 9127019141, Email: Ragerdi@razi.tums.ac.ir
-
Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
-
Atlasy, Nader
-
Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran
Abstract: Background: Diabetes Mellitus (DM), simply known as diabetes, refers to a group of metabolic diseases in which there are high blood sugar levels over a prolonged period. In this study, the feasibility and safety of intravenous transplantation of Very Small Embryonic Like stem cells (VSELs) were investigated for diabetes repair, and finally the migration and distribution of these cells in hosts were observed.
Methods: Mouse bone marrow VSELs were isolated by Fluorescent Activating Cell Sorting (FACS) method by using fluorescent antibodies against CD45, CXCR4 and Sca1 markers. Sorted cells were analyzed for expression of oct4 and SSEA1 markers with immunocytochemistry staining method. To determine multilineage differentiation, sorted cells were differentiated to Schwann, osteocyte and beta cells. Ten days after the establishment of a mouse model of pancreas necrosis, DiI-labeled VSELs were injected into these mice via tail vein. Pancreases were harvested 4 weeks after transplantation and the sections of these tissues were observed under fluorescent microscope.
Results: It was proved that CD45-, CXCR4+, and Sca1+ sorted cells express oct4 and SSEA1. Our results revealed that intravenously implanted VSELs could migrate into the pancreas of hosts and survive in the diabetic pancreas. In treated groups, blood glucose decreased significantly for at least two month and the weights of mice increased gradually.
Conclusion: This study provides a strategy for using VSELs for curing diabetes and other regenerative diseases, and the strategy is considered an alternative for other stem cell types.
Introduction :
Diabetes is a chronic metabolic disorder manifested by hyperglycemia due to a deficiency of insulin production by pancreatic β- cells. This can be a direct consequence of autoimmune destruction of β-cells, as seen in type 1 diabetes. Other types of diabetes, collectively known as type 2 diabetes, occur because of a combination of reduced insulin sensitivity (noninsulin dependent) and impaired β-cell function 1. Transplantation of pancreatic islets offers a direct treatment for type 1 diabetes and in some cases, insulin-dependent type 2 diabetes 1. Its widespread use is hampered by a shortage of donor organs 1; typically, the pooled islets isolated from two pancreases are enough to treat a single patient, so intensive research is being conducted to look for alternative sources of β-cells.
Since the enormous potential of stem cells was discovered, it was hoped that they would provide the most effective treatment for diabetes mellitus 2. Both embryonic stem cells (derived from the inner cell mass of a blastocyst) and adult stem cells (found in the postnatal organism) have been used to generate, surrogate β cells or otherwise restore β -cell functioning 3.
In 2006, Ratajczak et al identified Very Small Embryonic Like stem cells (VSELs) in the adult murine bone marrow and proved that these cells are pluripotent and express oct4, SSEA1 (stage-specific embryonic antigen 1) and CXCR4 4 (C-X-C chemokine receptor type 4). These cells, which are deposited during early gastrulation in developing tissues/organs have a small diameter (smaller than 6 µm) with large nucleus and a tiny rim of cytoplasm and play an important role in the turnover of tissue-specific/committed stem cells. Researchers have noticed that both in humans and mice, VSELs could be mobilized into Peripheral Blood (PB) and the number of these cells circulating in PB increases during stress and tissue/organ injuries (e.g., heart infarct, stroke) 5-8.
These cells are isolated form peripheral blood 7, umbilical cord blood 9, liver, and spleen too 10. The trafficking of VSELs is orchestrated by several chemotactic factors 10-13 that are upregulated in damaged tissues during organ injury, such as α-chemokine stromal derived factor (SDF-1), Hepatocyte Growth Factor/Scatter Factor (HGF/SF), Leukemia Inhibitory Factor (LIF), and Vascular Endothelial Growth Factor (VEGF).
VSELs have been proposed by some researchers as an alternative to embryonic stem cells. Very small embryonic like stem cells exist in the bone marrow of adult humans and mice, so they could obviate the ethical issues surrounding the use of human embryos. These cells don’t express Major Histocompatibility Complex (MHC1) and (MHC2) markers and besides don’t form teratomas 14.
If this process does indeed make it possible to develop these different types of cells, it would be a significant step toward resolving two major dilemmas facing scientists working on stem cell therapies for treating disease:
1. The ethical dilemma that has given rise to the debate over using human embryonic stem cells for research;
2. The immunological problems potentially associated with using stem cells from a donor embryo or person to treat disease-instead, treatments could be developed using the individual patient’s own bone marrow, eliminating the problem of donor tissue rejection 4.
In this project, it was purposed to evaluate the ability of mouse VSELs in cell therapy against type 1 diabetes. Bone marrow VSEL stem cells were sorted by FACS (Fluorescent Activating Cell Sorting) method against CD45 (Cluster of differentiation), CXCR4, and Sca1 antigens. Next, the expression of oct4 and SSEA1 in sorted cells was evaluated with ICC methods. To determine pluripotential capability of newly sorted cells, the cells were differentiated into Schwann, osteocyte and beta like cells. Cells of passage 3 were labeled with DiI and then injected into the tail vein of diabetic mice. Four weeks after transplantation, homing property of VSELs was assessed and blood glucose and weighs in this period were measured.
Materials and Methods :
The present study was performed in accordance with the guidelines of the Animal Care and Use Committee of the Tehran University of Medical Sciences.
Type of study: In this experimental study, there were 2 groups with 6 female mice (2 month old/NMRY) in each group. The first group was control, and the second was a diabetic group treated with VSELs.
Induction of diabetes in mice: Diabetes was induced by intraperitoneal high single dose (150 mg/kg) of streptozocin (sigma85882), and after one week, the mice which their non-fasting blood glucose was more than 400 mg/dl were included into the research. The mice were fasted for almost 4 hr prior to injection and supplied with 10% sucrose water overnight to avoid sudden hypoglycemia post-injection.
Assessment of diabetes and weights of the mice: Evaluation of rodent hyperglycemia is routinely performed by obtaining a drop of blood from the tail vein, placing it on a test strip, and measuring the glucose level with a standard patient glucometer (Bionime glucometer, model GM110). Before and after induction of diabetes, the weights of mice were recorded and compared with treated groups.
VSEL sorting: Twenty female mice (2 months old/NMRI) were sacrificed by cervical dislocation and under sterile conditions, bone marrow of femurs and tibias was flushed with KO/DMEM and gathered media centrifuged for 5 min at 1400 rpm. After that, cell suspension was adjusted to a concentration of 1-5´106 cells/ml in ice cold PBS, 10% FCS, 1% sodium azide 4. Primary antibody dilution was mixed with 1% BSA in PBS (phosphate buffered saline), then 0.1-10 µg/ml of the primary labeled antibody was added and incubated for at least 30 min at 4°C. In the next step, cells were washed 3´ by centrifugation at 400 g for 5 min and resuspended in 500 ml to 1 ml of ice cold PBS, 10% FCS, and 1% sodium azide. Next, the cells were kept in dark on ice or at 4°C and were analyzed 11. It was decided to sort a population of CD45-CXCR4+Sca1+ cells from murine bone marrow with BD FACS AriaII cell sorter devise. First, CD45- and CD45+ cells were separated by using anti-mouse CD45 (APC/Cy7 anti-mouse CD45 Catalog Number: 103115) and then from CD45- population, CXCR4+Sca1+ cells were isolated by using anti-mouse Sca1 FITC (Catalog number: ab 25031) and anti-mouse CXCR4 PE (Catalog number: 12‐9991) antibodies. The sorted cells were maintained in a proliferative state with LIF and cultured on Mouse Embryonic Fibroblast (MEF) cells to reach to passage 3.
MEF inactivation: MEF culture growth media (DMEM low glucose, FBS 10%, L-glutamine 5 mM, non essential amino acids, and penicillin/streptomycin) were exchanged with inactivation media, including MEF culture media plus mitomycin C (1 mg/ml) and PBS. The cells were incubated in 5% CO2 at 37°C for almost 3 hr, and then the media were expelled and washed 2 times with PBS. Now, the special media for maintaining VSEL stem cells were added to each flask.
VSEL proliferation: Mouse ES cells can be maintained in a proliferative, undifferentiated state in vitro by being grown on feeder layers of MEF cells. An alternative to culture on feeder layers is the addition of leukemia inhibitory factor to the growth medium.
For best results, VSEL stem cells were cultivated by two different ways. One group was proliferated on a feeder layer of mitotically inactivated MEF in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% FBS, L-glutamine (2 mM, Gibco), ß-mercaptoethanol (final concentration 5×105M), penicillin/streptomycin (100 U/ml penicillin and 100 µg/ml streptomycin (Gibco/BRL) and non essential amino acid (NEAA, stock solution diluted 1:100, Gibco). MEF cells secrete special chemical mediators that affect VSEL stem cells in a paracrine manner and inhibit differentiation. Another group was proliferated on coated dishes with the same culture media, plus LIF (10 ng/ml) (Sigma, L5158) 11, and the dishes were coated with 0/1% gelatin solution (Figure 1).
Immunocytochemical staining of enriched VSELs: The expression of each antigen was examined in cells from two independent sorts. After passage 1, the CD45-/Sca1+ /CXCr4+ sorted cells were fixed for 2 hr at room temperature with 4% paraformaldehyde and then incubated at room temperature for 15 min with 1% Triton ´-100/phosphate-buffered saline (PBS). Cells were washed three times in PBS and blocked at 37oC for over 3 hr with 4% normal goat serum (Chemicon). Subsequently, cells were incubated at 4°C overnight with rabbit polyclonal primary antibody to oct4 (1:200, Abcam18976, USA), and mouse monoclonal primary antibody to SSEA1 (1:200, Abcam16285, USA). Cells were washed three times in PBS and incubated at 37oC for 2 hr with FITC goat anti rabbit (ab98511) and PE goat anti mouse secondary antibodies (1:500 in 1% normal goat serum in PBS). Unbound secondary antibodies were removed in three washes with PBS. Nuclei were identified by DAPI (Invitrogen) staining at a dilution of 1:10,000 at room temperature for 5 min. Images were acquired using an inverted microscope (Olympus, IX71, and Japan) (Figure 2).
Multilineage differentiation of VSEL stem cells
Transdifferentiation of VSEL stem cells into schwann cells: VSEL cells at passage 3 were detached from culture dishes and plated at the concentration of 1×105 cells/cm2 on 24-well culture plates, cells were incubated in culture medium containing 1 mM beta mercaptoethanol without serum for 24 hr. The culture media were then replaced with new media containing 10% FBS and 35 ng/ml all trans-retinoic acid (Sigma, USA). Three days later, cells were finally transferred to culture media containing 10% FBS and trophic factors of 5 mM forskolin (FSK) (Calbiochem, CA), 10 ng/ml recombinant human basic Fibroblast Growth Factor (bFGF) (Peprotech, UK), 5 ng/ml platelet-derived growth factor-A (PDGF) (Peprotech, UK), and 200 ng/ml Heregulin-b1-EGF-domain (HRG) (R&D systems, USA) and cultured for 10 days.
Immunostaining of schwann cells: Differentiated Schwann like cells cultured on chamber slides (Lab-Tek, Denmark) were fixed in 4% (w/v) paraformaldehyde at 4°C for 20 min. Cell nuclei were labeled with 6 diamidino-2-phenylindole dihydrochloride (DAPI, Sigma-Aldrich) (1 µg/ml) for 60 min at room temperature. Cells were then incubated overnight at 4°C with primary antibodies to S100 (rabbit polyclonal; 1:200; Dako, Denmark). The following day, slides were incubated for 2 hr with FITC-conjugated secondary antibody (horse anti-mouse or goat anti-rabbit; 1:100; Vector Labs., USA). Slides were examined under a fluorescence microscope (Olympus BX60) (Figure 3).
Osteogenic differentiation: Sorted cells were treated with osteogenic medium (OS) containing 0.05 mM ascorbate, 1 µM dexamethasone and 10 mM b-glycerophosphate for 4 weeks.
Confirmation of osteogenic differentiation: Osteogenesis was confirmed by means of Von Kossa staining. This technique is for demonstrating deposits of calcium or calcium salt. For Von Kossa silver nitrate staining method, cultures were fixed in cold methanol for 15-20 min. After rinsing, the fixed plates were incubated with 5% silver nitrate solution under UV light using 2 cycles of auto-crosslink (1200 mj×100) in a UV Stratalinker (Strategene, La Jolla, CA). Mineralized nodules were seen as dark brown to black spots (Figure 4).
Beta cell induction: Activin A and nicotinamide were selected as main differentiation factors. With two step protocols 4, VSEL stem cells were differentiated into beta like cells.
In the first step, DMEM/F12 with 4 mM/l glutamine, 4.5 g/l glucose, 1% heat in activated FBS and 50 ng/ml of recombinant human Activin A were used.
In the second step, after 72 hr, DMEM/F12 with 4 mM/l glutamine, 4.5 g/l glucose, 5% heat inactivated FBS in the presence of N2 supplement-A,B27 supplement, and 10 mM nicotinamide were used. Medium was changed every other day and beta like cells appeared after 24 days 4.
Immunocytochemical analysis of differentiated beta like cells: For this purpose, two antibodies were used against antigens of beta like cells; the first was rabbit polyclonal to PDX1primary antibody (ab47267) and goat polyclonal secondary antibody to rabbit IgG-H&L (FITC) (ab97050) for PDX1 detection. The second was anti-glucose transporter GLUT2 primary antibody (ab54460) and goat polyclonal secondary antibody to rabbit IgG-H&L (FITC) (ab97050) for GLUT2 detection. Detailed process of the ICC was similar to ICC for VSEL stem cells described earlier (Figure 5).
Cell Labeling and transplantation: Ten days after induction of diabetes, injection of labeled VSEL stem cells into the tail vein of diabetic mice was done. DiI (CellTrackerTM CM-DiI; C7000, V22888) is a long-chain carbocyanine membrane probe and particularly suitable for long-term labeling and tracking of cells 10. Immediately before labeling, 1-2 mg/ml solution stock was diluted into a suitable medium such as a Hanks’ Balanced Salt Solution (HBSS). Suitable working concentrations generally range from 1-2 µM but may vary considerably depending on the application. VSEL stem cells of passage 3 were trypsinized, washed, and incubated with DiI at a concentration of 1×106 cells per ml with 5 µl of DiI/ml in proliferation medium for 30 min in a humidified incubator at 37°C in 5% CO2 prior to the transplantation. After being washed three times with Hanks' balanced saline solution (HBSS), DiI-labeled VSEL cells were resuspended in HBSS at 5´106 cells per 50 µl.
VSEL Administration: VSEL stem cells (1×106) were labeled with 8 mg/ml DiI (Invitrogen, Eugene, OR) and resuspended in 0.2 ml of 5% mice plasma and preserved on ice until the time of injection. After labeling, the cells were promptly administered via the tail vein in lightly anesthetized mice. Untreated animals received 0.2 ml of 5% mice plasma.
Assessment of homing property in VSEL stem cells: After one month, the mice were euthanized and the pancreas harvested and fixed in 4% formaldehyde overnight, and then transferred into 10% formalin for subsequent days. Then the tissues were prepared for sectioning and further assessments. Evaluation of the DiI-labeled cells in tissue slides was performed with inverted microscope and recorded with the image. The viability of the cells in the pancreas was important (Figure 6).
Statistics analysis: Data are presented as means±standard deviation (SD) with n indicating the number of mice. Statistical analysis was performed using Student’s t test. Values of p=0.0002 were considered statistically significant.
Results :
Cell sorting: In multicolor flow cytometric sorting analysis (FACS) of mouse bone marrow cells, from CD45 negative population, Sca1 and CXCr4 positive cells were gated. In a 3 color analysis, APC-Cy7 anti-mouse cd45 antibody was used for isolation of CD45 negative and positive cells, then from CD45 negative population, Sca1 and CXCr4 positive cells with anti-mouse Sca1 FITC and anti-mouse CXCr4 PE antibodies were sorted (Figure 7).
Proliferations of VSEL stem cells: VSEL stem cells had a strong self-renewal capacity and to avoid spontaneous differentiation, LIF was crucial. For prevention of colony formation, cell culture media were changed every day (Figure 1) with every other day passages. At first, the proliferation of VSEL cells with 1×104 cells was done and they were maintained in the proliferative state with LIF. The cells were cultured on mitomycin-C treated MEF feeders and they had a better situation regarding proliferative ability and had a better self renewal in comparison to the cells cultured on gelatin coated dishes without any MEF cells.
ICC of sorted cells: Immunocytochemistry of CD45-, Sca1+, and CXCR4+ cells of passage 3 revealed that the cells expressed SSEA1 and oct4 (embryonic stem cell markers) vigorously (Figure 2).
Assessment of pluripotential capability of sorted cells: The results of differentiation showed that VSEL sorted cells were pluripotential and capable to differentiate to all 3 layer derivatives, like Schwann, osteocyte, and beta like cells (Figures 3, 4 and 5).
Assessment of homing property in VSEL stem cells: Evaluation of paraffin sections in pancreas of mice that received labeled VSEL stem cells via tail vein showed that injected labeled cells migrated to inflamed pancreas of diabetic mice and survived at least 1 month until the time of pancreas harvesting. Labeled cells were detected with inverted fluorescent microscope and then DAPI was applied for counterstaining of nucleus (Figure 6).
Comparison of blood sugar in control and diabetic groups: Within a week after VSEL administration, blood glucose levels significantly decreased until reaching almost euglycemic values a month later. Hyperglycemia correction lasted at least for 2 months (Figure 8).
The results of assessment of mean body weights: One week after STZ injection and induction of diabetes, mice showed substantial weight loss, and one month after cell therapy the weights of mice were corrected. The results were analyzed with GraphPad Prism 5, and the obtained data were statistically significant (Figure 9) (p=0.0002).
Discussion :
Nowadays, intravenous transplantation has been a common strategy in the treatment of many diseases, including severe autoimmune diseases 15-18, myocardial infarction 19-21, liver failure 19, and trauma 22-25. Recently, in order to improve the efficacy of stem cell transplantation, committed stem cells are isolated and purified, and single lineage stem cell transplantation is then performed 26.
One type of stem cell that has been used to treat diabetes mellitus and investigated extensively in animal models is Mesenchymal Stem Cells (MSCs) 27. Almost all research on MSC transplantation shows that in vitro or in vivo transplantation of MSCs results in reduction of blood glucose levels, weight gain and increased longevity 2.
MSCs have been successfully differentiated into IPCs in vitro and can reduce blood glucose levels in both animals and humans after transplantation. In vitro differentiation of MSCs into IPCs requires certain substances combined with medium stress. Most successful protocols for the differentiation of MSCs into IPCs used nicotinamide and/or exendin-4 inducers. Changes in the glucose concentration within the culture medium are necessary to trigger this process. MSCs are commonly cultured in low glucose medium to initiate differentiation before they can be induced to differentiate into IPCs by nicotinamide. In some studies, Epidermal Growth Factor (EGF) was added to the culture medium during the IPC maturation phase in addition to nicotinamide.
MSCs can be obtained from human umbilical cord blood 28-30, banked human umbilical cord blood 31, placenta 32, bone marrow 32-34, menstrual blood 35, amniotic fluid 36, Wharton’s jelly 37,38, amnion 39, and adipose tissue 40. Dong et al 41 used bone marrow MSCs to treat STZ induced diabetic rats. They transplanted MSCs intravenously and after 45 days, the blood sugar of diabetic rats decreased significantly.
Safety is a critical concern of intravenous transplantation of MSCs. The main concern in this study was whether intravenous transplantation of MSCs can cause immunological rejection or not. The answer is yes, so VSELs instead of MSCs were used to treat diabetes. In the present study, no local or systematic manifestations of acute and chronic toxic reactions and graft versus host disease were observed during and after MSCs transplantation.
However, as MSC therapy gains popularity among practitioners and researchers, there have been reports on the adverse effects of MSCs especially in the context of tumor modulation and malignant transformation. These cells have been found to enhance tumor growth and metastasis in some studies and have been related to anticancer-drug resistance in other instances 42,43. In addition, various studies have also reported spontaneous malignant transformation of MSCs. The mechanism of the modulatory behavior and the tumorigenic potential of MSCs warrant urgent exploration, and the use of MSCs in patients with cancer awaits further evaluation. However, if MSCs truly play a role in tumor modulation, they can also be potential targets of cancer treatment. For the reasons cited and to reach better results, researchers examine other stem cell types.
Using ESCs to treat diabetes mellitus is limited because of high levels of tumor formation and for this reason there are a few researches using the ESCs for treating diabetes mellitus 2. In this paper, the use of VSELs could conquer against these obstacles. Stem cell transplantation strategies are various; cells can be grafted underneath the kidney capsule 44-48, delivered via intra-peritoneal injection 48 or intra-portally 49, grafted into the liver 50 or injected into the tail vein 51- 53.
However, there is little research comparing the efficiency of these methods. Zalzman et al 54 showed that transplantation of stem cells into the liver produces better results than transplantation into the renal capsule. VSELs have homing capability, so intravenous injection method was used for cell therapy against diabetes type1 in our project.
Although diabetes mellitus is caused by destruction of the beta cells within the pancreatic islets, no studies have attempted direct transplantation into the pancreas. This is because the pancreas is a very sensitive organ and is vulnerable to mechanical intervention.
In another attempt, Kodama et al transplanted pancreatic cell ontogeny within ESCs into the renal capsule of STZ induced diabetic mice which resulted in pancreatogenesis in situ or beta cell neogenesis. Twenty-one days post transplantation, PDX-1+ pancreatic foci appeared in the renal capsule, which expressed exocrine enzymes (amylase) and endocrine hormones (insulin, glucagon, and somatostatin). These multi-hormonal endocrine cells, characteristic of beta cell regeneration, indicated possible divergence from embryonic islet cell development 29. In another study, Kodama et al showed that transplanted ESCs could migrate into the injured pancreas. Cell tracing analysis showed that significant beta cell neogenesis occurred 2 to 3 weeks after injury 30.
As an alternative to ESCs, researchers used VSELs to cure diabetes. This way, first the ethical concerns in relation to embryonic stem cells will be removed and second VSELs do not express MHC-1 and HLA-DR antigens 31 and they don’t form teratomas.
Ratajczak et al, succeeded to differentiate murine VSEL stem cells into pancreatic cells that expressed insluin1 and insulin 2 markers. However, they didn’t explain whether differentiated cells could produce insulin in response to glucose stimulations or no. Moreover, they didn’t examine new pancreatic cells in vivo 4. In another research, Ratajczak et al proved that VSEL cells were mobilized into injured pancreatic tissue and they contribute to beta cell regeneration. Transplantation of BM-derived cells improves the function of injured pancreas, although the response is not sufficient to restore sustained normoglycemia 34.
VSEL stem cells are CXCR4 positive and respond robustly to an SDF-1 gradient 14. It is well documented that damaged tissues upregulate the expression of several chemoattractants of TCSC 10,11. In addition to SDF-1, other factors such as VEGF, HGF/SF, LIF or FGF-2 are upregulated in damaged organs as well. Hypoxia regulated/induced transcription factor (HIF-1) plays an important role in expression of several of these factors 10,11.
In our research, mice bone marrow VSELs were isolated with multicolor fluorescent activating sorting method and cultured those to reach to passage 3. It was recognized that these cells were smaller than 6 µm with large nucleus and tiny rim of cytoplasm. Immunocytochemistry revealed that sorted cells expressed SSEA1 and oct4, as researchers proved it before 4. Also, pluripotential capability of VSEL sorted cells was analyzed and differentiation of the cells into astrocyte, osteocyte and beta like cells could be done. This finding approved that these cells undergo unlimited self-renewal and retain the pluripotency to differentiate into all cell lineages in the body.
To prove the migration and homing ability, the labeling of VSELs with DiI was performed and 10 days after induction of diabetes, they were injected intravenously, because SDF-1 significantly increased in the pancreas after damage 32, peaking on day 10. Some researchers didn’t pay attention to this timing point and failed in their protocols 19,20.
After one month, homing property of VSELs was assessed by using immunohistochemistry technique and it proved that labeled cells were seen in the pancreas. Evaluation of blood sugar and weight of the mice showed that blood sugar was too close to euglycemic values and weights of the mice increased in comparison to the time before cell therapy.
Conclusion :
Safety is a critical concern of intravenous transplantation of stem cells, and the main concern is whether intravenous transplantation of VSELs can cause immunological rejection or not. In the present study, no local or systematic manifestations of acute and chronic toxic reactions and graft versus host disease were observed during and after VSELs transplantation.
Although our results showed that intravenously implanted allogeneic VSELs could directionally migrate to pancreas and survive, especially in the diabetic pancreas, the mechanisms underlying the directional migration of VSELs should be further studied. Besides, the efficacy of intravenous transplantation of VSELs in the treatment of diabetes should also be further confirmed.
It can be expected that diabetes mellitus will be successfully treated using stem cell therapy in the near future. However, questions regarding the survival of the cells after grafting and improvements in the vitality and maintenance of cellular function after transplantation remain to be answered.
Acknowledgement :
This project was financially supported by the Tehran University of Medical Sciences (TUMS), Faculty of Medicine. Our special thanks go to chairman of Royan Institute, Professor H. Gurabi, and Dr A.H. Shahverdi for permitting us to use BD FACS AriaII cell sorter devise. Also, our thanks go to Mr. Janzamin and Mrs. Khosravani in relation to work with FACS devise and their helps to sort the cells. The authors declare that there is no conflict of interests regarding the publication of this article.
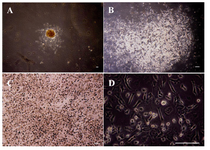
Figure 1. (A-D): undifferentiated VSELs grown on MEF cells, colony formation of VSELs after 3 days, Bar=100 µm. A) A colony of VSEL stem cells (40x); B) A colony of VSEL stem cells (100x), round bright cells are VSELs; C) a colony of VSEL stem cells (200x); D) A colony of VSEL stem cells (400x) (image was taken by Olympus IX71 inverted microscope). (Image was taken by Olympus BX51 microscope).
|
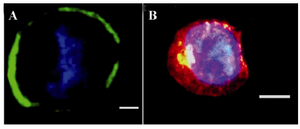
Figure 2. Immunofluorescence staining of VSEL stem cell. CD45-, Sca1+, and CXCR4+ cells are SSEA1+ and express oct4; A) Oct4 (green) expression; B) SSEA1 (red) expression. Nuclei visualized after DAPI staining (40x) (image was taken by Olympus IX71 inverted microscope).
|

Figure 3. Immunofluorescence staining of Schwann like cells, these cells express S100; A) Nuclei visualized after DAPI staining; B) Merged; C) (20x).
|
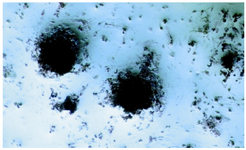
Figure 4. Von Kossa staining after osteogenic differentiation: Staining for mineral deposition was performed for osteocyte like cells. This staining was done only for demonstration of osteogenic differentiation (40x).
|
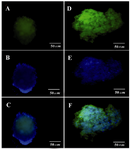
Figure 5. ICC for Glut2 and PDX1 markers in differentiated beta like cells. A) Glut2 expression was indicated by green fluorescence; B) DAPI nuclear counterstain in blue; C) Merged picture (40x); D) PDX1 was indicated by green fluorescence; E) DAPI nuclear counterstain in blue; F) Merged picture (40x).
|
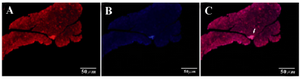
Figure 6. IHC of harvested pancreas after 1 month. After tail vein injection DiI-labeled cells migrate to pancreas in diabetic mice (white arrow). A) DiI-labeled cells after migration (20x); B) DAPI for nuclear staining (20x); C) Merged.
|
![Figure 7. [FACS analysis of mouse bone marrow cells]. Erythrocytes were removed by hypotonic lysis and bone marrow cells were stained with CD45, Sca-1 and CXCr4. VSEL stem cells were sorted by BD FACS Aria II cell sorter, following immunofluorescence staining for CD45, Sca-1 and CXCr4. Panel A: Gated cell population of interest. Panels B and C: Bone marrow mononuclear cells visualized on dot plots showing their FSC and SSC signals related to the size and granularity of the cell, respectively. Panel D: Separation of CD45 negative and positive cells. Panel E: CD45 negative cells gated based on Sca1 and CXCr4 positivity (Q2 area)].](Images/Articles/197/f7_small.png)
Figure 7. [FACS analysis of mouse bone marrow cells]. Erythrocytes were removed by hypotonic lysis and bone marrow cells were stained with CD45, Sca-1 and CXCr4. VSEL stem cells were sorted by BD FACS Aria II cell sorter, following immunofluorescence staining for CD45, Sca-1 and CXCr4. Panel A: Gated cell population of interest. Panels B and C: Bone marrow mononuclear cells visualized on dot plots showing their FSC and SSC signals related to the size and granularity of the cell, respectively. Panel D: Separation of CD45 negative and positive cells. Panel E: CD45 negative cells gated based on Sca1 and CXCr4 positivity (Q2 area)].
|
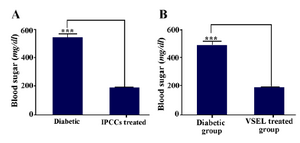
Figure 8. A) Average non-fasting glucose levels of control and experimental diabetic mice. B) Average non-fasting glucose levels of diabetic group, before and after treatment (the mean blood sugar of treated diabetic mice is calculated 3 weeks after cell therapy with VSELs). Asterisks in figure denote statistical significance, (p= 0.0002).
|
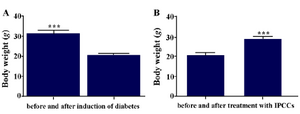
Figure 9. A) Mean body weight of mice before and after (3 weeks) induction of diabetes; B) Mean body weight of diabetic mice before and after (3 weeks) treatment with VSEL stem cells. Body weight of diabetic mice differed significantly before and after treatment. Asterisks in figure denote statistical significance (p=0.0002).
|
|