Production and Characterization of Monoclonal Antibodies against Human Prostate Specific Antigen
-
Bayat , Ali Ahmad
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Ghods, Roya
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Department of Molecular Medicine, School of Advanced Medical Technologies, Tehran University of Medical Sciences, Tehran, Iran
-
Shabani, Mahdi
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Mahmoudi, Ahmad Reza
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Yeganeh, Omid
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Hassannia, Hadi
-
Department of Immunology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran
-
Sadeghitabar, Ali
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
Balay-Goli, Leila
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
-
 Jeddi-Tehrani, Mahmood
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel:+98 21 22432020, Email:mahjed@yahoo.com
Jeddi-Tehrani, Mahmood
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran, Tel:+98 21 22432020, Email:mahjed@yahoo.com
-
Monoclonal Antibody Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Background: Prostate Specific Antigen (PSA) is an important laboratory marker for diagnosis of prostatic cancer. Thus, development of diagnostic tools specific for PSA plays an important role in screening, monitoring and early diagnosis of prostate cancer. In this paper, the production and characterization of a panel of murine monoclonal antibodies (mAbs) against PSA have been presented.
Methods: Balb/c mice were immunized with PSA, which was purified from seminal plasma. Splenocytes of hyperimmunized mice were extracted and fused with Sp2/0 cells. By adding selective HAT medium, hybridoma cells were established and positive clones were selected by ELISA after four times of cloning. The isotypes of produced mAbs were determined by ELISA and then purified from ascitic fluids using Hi-Trap protein G column. The reactivities of the mAbs were examined with the purified PSA and seminal plasma by ELISA and western blot techniques. Furthermore, the reactivities of the mAbs were assessed in Prostate Cancer (PCa), Benign Prostatic Hyperplasia (BPH) and brain cancer tissues by Immunohistochemistry (IHC).
Results: Five anti-PSA mAbs (clones: 2G2-B2, 2F9-F4, 2D6-E8, IgG1/К) and clones (2C8-E9, 2G3-E2, IgG2a/К) were produced and characterized. All mAbs, except 2F9-F4 detected the expression of PSA in PCa and BPH tissues and none of them reacted with PSA in brain cancer tissue in IHC. Besides, all mAbs could detect a protein band around 33 kDa in human seminal plasma in western blot.
Conclusion: These mAbs can specifically recognize PSA and may serve as a component of PSA diagnostic kit in various biological fluids.
Introduction :
Prostate Cancer (PCa) is the most common cancer among men and the third leading cause of cancer death in developed countries 1. Prostate Specific Antigen (PSA) is a serine protease with a chymotrypsin-like activity 2. PSA is a 33 kDa glycoprotein that is secreted by prostate epithelial cells into prostatic ducts as a proenzyme with 244 amino acids and then activated by cleavage of seven N-terminal amino acids 3. It is a major protein in semen at concentrations of 0.2-5 mg/ml that liquefies the seminal coagulum after ejaculation 4,5. In healthy individuals, minute amounts of PSA leak into blood vessels, whereas high serum concentrations of PSA can be detected in patients with PCa, Benign Prostatic Hyperplasia (BPH), and bacterial prostatitis 3,6. PSA is used as a serum marker for screening, monitoring and early diagnosis of prostate cancer 7,8. In healthy men, the majority of the serum PSA forms covalent complexes with two predominant serine protease inhibitors, α1-antichymotrypsin and α2-macroglobulin that cause the inactivation of the chymotrypsin-like activity of PSA 9. In semen, about 65% of PSA has enzymatic activity, and 35% seems to be inactive which is due to an internal cleavage of the peptide chain 10,11. Interestingly, PSA is also found to create a 90 kDa complex form with Protein C Inhibitor (PCI) in semen 12,13. PCI, a member of the serine protease inhibitor (serpin) family, is a 57 kDa single-chain glycoprotein with 387 amino acids which is structurally similar to α1-antichymotrypsin 13. Measuring PSA is routinely used for early diagnosis, screening and management of PCa 14. This study aimed to produce and characterize murine anti-human PSA antibodies which will be applied for development of an ELISA-based assay for measurement of PSA in the future.
Materials and Methods :
Purification of PSA: PSA was purified from human seminal fluid by PSA affinity chromatography method. In this regard, anti-PSA mAb was coupled to CNBr-activated Sepharose 4B (GE Healthcare, Uppsala, Sweden). The seminal fluid was diluted with PBS in 1:10 ratio, centrifuged at 1200 g for 10 min and then filtered through 0.45 µm filters (Orange Scientific, Braine-1' Alleud, Belgium). The cleared seminal fluid was loaded on column. Captured PSA proteins were eluted by glycine-HCl (0.1 M, pH=2.7) and then immediately dialyzed against PBS (pH=7.5) at 4°C overnight. The purity of purified PSA was analyzed by SDS-PAGE.
Immunization of mice: Female Balb/c mice aged 6 to 8 weeks (Pasture Institute, Tehran, Iran) were immunized intraperitoneally with 50 µg of highly-purified PSA emulsified with complete Freund's adjuvant (Sigma-Aldrich, Wisconsin, USA) followed by four booster injections of PSA emulsified with incomplete Freund's adjuvant (Sigma-Aldrich). One week after the last immunization, blood was collected from the tail vein for determination of anti-PSA antibody titers by enzyme-linked immunosorbent assay (ELISA). Three days before the cell fusion, 20 µg of PSA (without any adjuvant) were injected intravenously 15,16. The use of animals were approved by the ethical committee of Avicenna Research Institute.
Hybridoma cell generation: Anti-PSA monoclonal antibodies (mAbs) were generated as described elsewhere 15. Briefly, murine myeloma cell line, Sp2/0, was cultured in RPMI-1640 medium (Gibco, Gran Island, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco) and used as the fusion partner. Splenocytes from the immunized mouse were mixed with Sp2/0 cells at a ratio of 1:5. The cell mixture was washed twice with pre-warmed RPMI-1640 (37°C). Fusion was then performed using pre-warmed 50% polyethylene glycol (PEG) 1500 (Sigma-Aldrich). Selective HAT medium (Sigma-Aldrich) was then added to the wells for selection of hybridoma cells. The reactivities of hybridoma supernatants were checked by ELISA method as described below. Finally, positive hybridoma cells were cloned four times by limiting dilution to select the stable hybridomas.
ELISA: For titration of ant-PSA in the mice sera and screening of anti-PSA producing hybridoma cells, purified PSA and human seminal plasma were separately coated at 10 µg/ml and 1:2000 dilution in 96-well ELISA plates, respectively (Nunc, Roskilde, Denmark). The plates were incubated at 37°C for 1 hr followed by incubation at 4°C overnight. The wells were washed 3 times with PBS containing 0.05% Tween 20, (PBS-T) for 3 min and then blocked with 2.5% skim milk in PBS-T at 37°C for 1 hr. Serial dilutions of mouse sera and hybridoma supernatants were added to the wells and subsequently incubated for 1 hr. After washing, horseradish peroxidase (HRP)-conjugated rabbit anti-mouse Ig (1:1000) (Avicenna Research Institute, Tehran, Iran) was added to the wells and incubated for 1 hr at 37°C. Then, 50 µl of ortho-phenylenediamine (OPD) or tetramethylbenzidine (TMB) substrates (Sigma-Aldrich) were added to each well and the plates were incubated at room temperature in the dark. After 15 min, the reaction was stopped by adding 15 µl stopping solution (20% H2SO4) to each well. The optical density (OD) was measured at appropriate wavelength by an ELISA reader (BioTek, Winooski, VT, USA). The explained procedure was applied for isotype determination of produced anti-PSA mAbs, except that goat anti-mouse IgG1, IgG2a, IgG2b, IgG3, IgM and IgA (1/1000 dilution) (Sigma-Aldrich) were coated in the 96-well plates 15. In addition, the captured anti-PSA mAbs were detected by HRP-conjugated rabbit anti-mouse Ig (1:1000).
Ascitic fluid production: Briefly, Balb/c mice were peritoneally injected with 0.5 ml 2,6,10,14-Tetramethyl-pentadecane (Pristane) (Sigma-Aldrich). After one week, 5×106 hybridoma cells were inoculated into peritoneal cavity of the mice. The ascitic fluids were harvested after 7-10 days 17.
Anti-PSA mAbs purification: Anti-PSA mAbs were purified by Hi-Trap protein G column (GE Healthcare) according to the manufacturer’s instructions. Briefly, the ascitic fluids were diluted 1:5 with PBS and filtered through 0.45 µm filters (Orange Scientific). The diluted ascetic fluids were passed through the column and unbound proteins washed out with PBS. The captured antibodies were eluted by glycine-HCl (0.1 M, pH=2.7) and then dialyzed against PBS (pH=7.5) at 4°C overnight 15. The concentrations of purified mAbs were calculated by optical densitometry method at 280 nm.
Assessment of mAb reactivities by western blot analysis: Human seminal plasma samples were run on a 12% SDS-PAGE gel. After electrophoresis, resolved proteins were transferred onto PVDF membranes (Millipore corporation, Billerica, Massachusetts, USA). The membranes were blocked with 5% skim milk in PBS-T at 4°C overnight. After gentle washing with PBS-T, all purified mAbs were added to the membrane and incubated for 1.5 hr at room temperature. Purified normal mouse Ig at the same concentration of anti-PSA mAbs was used as a negative control in the experiments. After extensive washing with PBS-T, the membrane was incubated with HRP-conjugated rabbit anti-mouse Ig (Avicenna Research Institute) (1:2500) for 1 hr at room temperature followed by washing with ECL chemiluminescence detection system (GE Healthcare).
Immunohistochemistry (IHC): Formalin-fixed paraffin-embedded human PCa and BPH tissues (Department of pathology, Khatam al anbia Hospital, Tehran, Iran) were checked for PSA expression by IHC method. For assessing anti-PSA mAbs specificity, brain cancer sections were served as PSA-negative tissue in our experiments 18. In the next step, 4 µm of thick tissue sections were deparaffinized with xylene, then rehydrated in decreasing concentrations of ethanol. Antigen retrieval was performed in citrate buffer at 94°C for 30 min in water bath. After washing with Tris-buffered saline (TBS), endogenous peroxidase activity was quenched by addition of hydrogen peroxidase (3%) in TBS for 10 min. Nonspecific binding was blocked with 5% goat serum in TBS-Tween 0.05% for 20 min and slides were then tilted and incubated with anti-PSA mAbs (10 µg/ml) in TBS containing 1% BSA for 60 min. Furthermore, irrelevant isotype-matching antibody (an anti-idiotype mAb, clone 2F9-G5 (IgG1/κ)) was included in the experiments. The sections were then washed with TBS containing 1% BSA and incubated with EnVision detection system (Dako, Copenhagen, Denmark) for 20 min. Color was developed using 3, 3'-diaminobenzidine (DAB) substrate solution (Sigma-Aldrich) and the slides were counterstained with Mayer’s hematoxylin 19.
Results :
Purification of PSA: The PSA was successfully purified form seminal plasma. Detection of purified PSA in SDS-PAGE demonstrated a single specific band at ~33 kDa (Figure 1). The PSA was highly purified with no contamination with other proteins of seminal fluid.
Production of anti-PSA mAbs and assessment of their reactivities by ELISA: After immunization of Balb/c mice, the titers of anti-PSA antibodies in the mice sera were detected by indirect ELISA. Mouse 1 with higher titer of anti-PSA antibody was selected for hybridoma production (Figure 2). After cell fusion, supernatants of growing hybridoma cells were screened based on their reactivity with the purified PSA by ELISA. After four times of cloning, five hybridoma cells named 2G3-E2, 2F9-F4, 2G2-B2, 2D6-E8 and 2C8-E9 were produced. All five anti-PSA mAbs were produced in ascitic fluids and purified.
Characterization of produced anti-PSA mAbs: First of all, the isotypes of all specific mAbs were determined by home-made ELISA as described in methods section. Our results showed that the isotypes of 2G2-B2, 2F9-F4 and 2D6-E8 were IgG1/k and those of 2G3-E2 and 2C8-E9 were IgG2a/k (Tables 1 and 2). In the next step, reactivities of the purified mAbs were assessed by an indirect ELISA on purified PSA (Figure 3A) and also seminal fluids (Figure 3B). Our findings showed that all produced mAbs specifically recognized PSA in both purified PSA and crude mixture of seminal fluids. The highest reaction was observed for clone 2C8-E9 which recognized the target at lowest concentration of the mAb (9.7 ng/ml). Besides, reactivities of the mAbs were checked in western blot on human semen samples. In this regard, all anti-PSA mAbs detected a 33 kDa protein band in human semen corresponding to free PSA. Two distinct bands were also observed at 28 and 90 kDa (Figure 4). For more characterization, the mAbs were applied for staining of PSA expressing tissues, PCa and BPH, in IHC experiments. Although all clones except 2F9-F4 strongly reacted with PCa and BPH tissues, no reactivity was observed in brain cancer sections as PSA-negative control tissue (Figure 5).
Discussion :
Over the past decade, PSA has been introduced as the most important biomarker in urologic oncology. Currently, PSA is widely used as a biomarker for scre-ening, monitoring and early diagnosis of PCa in men 1. Indeed, PSA immunoassays are routinely performed for measuring PSA concentration and monitoring PCa progression 20.
In the present study, PSA was used which was purified from seminal plasma for production of mAbs. The purity of PSA was determined by SDS-PAGE which showed a single band of about 33 kDa. Moreover, the identity of the purified PSA was confirmed by a commercial PSA measurement electrochemiluminescent kit, providing further evidence that the purified protein was PSA.
In this study, five anti-PSA mAbs with IgG1 and IgG2a subclasses were produced. The specificities of the anti-PSA mAbs were checked by ELISA, western blot and IHC assays. It should be noted that some of the antibodies might be from the same hybridoma clones but at least those that have different isotypes must be from distinct clones. However, the antibodies with the same isotypes might be from a single hybrid-oma clone, although there is still the possibility that they come from different clones. In this regard, blocking assay may provide some solution. If the antibodies do not block each other, there is a bigger chance that they come from different clones. Of course in this regard, different affinities might add to the controversy. On the other hand, if the antibodies block each other, they may be from the same mother clone. In this case, one should also consider the possibility that different antibodies recognizing very close epitopes might block each other by steric hindrance and still be from different clones. Furthermore, 2G3-E2, 2F9-F4, 2G2-B2 and 2D6-E8 mAbs are from one fusion and 2C8-E9 mAb is from a second fusion. In addition, 2G3-E2 mAb had a different isotype from that in the other three mAbs from the same fusion and 2F9-F4 mAb was negative in IHC compared to others.
The reactivities of the mAbs with PSA were detected with both diluted seminal fluid and purified PSA in ELISA. Likewise, western blot analyses indicated that the produced mAbs recognized a 33 kDa band with free PSA which is the main isoform of PSA as reported in human semen 3. The detection of the 28 kDa band by the mAbs is in line with the fact that PSA molecule has three endoproteolytic cleavage sites and auto catalytic activity 21 which results in multiple isoforms with different sizes 22,23. Detection of the 90 kDa band in seminal fluid may also be justified by PSA and PCI complex formation in human seminal fluid 13.
The produced anti-PSA mAbs reacted differently with PSA-PCI complex. One view for interpreting these results may be the difference in PCI amounts in different samples. Especially, it is worth to note that our seminal fluid samples were obtained from an infertility clinic, and based on Kise et al’s report, PCI levels could be extremely low in azoospermia 24. Thus, different concentrations of PCI in different samples could originate from the sources of samples. In addition, it could result from different reactivities of our mAbs with free PSA or PSA-PCI complexes that may lead to different densities of the observed bands. It is important to note that seminal fluid is mainly composed of free PSA 4. Therefore, the band densities of free PSA (~30 kDa) were stronger than the bands related to PSA-PCI (90 kDa). Again the differences in reactivit-ies of our mAbs with free PSA and PSA-PCI could originate from their affinities to or specificities of free PSA or PSA-PCI.
In addition to ELISA and western blot experiments that revealed functionality of our produced anti-PSA mAbs, the reactivities of the mAbs were also studied in IHC. In this regard, our findings demonstrated that all mAbs (except 2F9-F4) could detect the PSA in BPH and PCa tissues in IHC. Notably, IHC is a multi-step process with fixation and antigen retrieval which can cause molecular modifications leading to loss of mAb reactive epitopes 25. Hence, it is possible that the 2F9-F4 reactive epitope is subjected to such alterations. Furthermore, none of the mAbs showed any reactivity with the PSA negative brain cancer tissue implying their specificities for PSA.
In line with the present study, Baumgart et al produced anti-PSA mAbs by immunizing mice with PSA antigen and Freund’s adjuvant to enhance the immune response 26. However, genetic immunization has not been so efficient as its protein counterpart 8.
Wu et al found multiple isoforms of PSA molecule which were able to form complexes with protease inhibitors 22. Black et al produced a panel of anti-PSA mAbs which recognized both free PSA and PSA-ACT and 1 mAb detected only free PSA 20.
IHC analysis of PSA expression in tumor tissues has been extensively used in the identification of the prostatic malignancies. In this regard, Lamp et al. assessed mAbs reactivities with PCa tissue samples by IHC 27. Liu et al also showed immunohistochemical reactivity of IgG mAbs with PCa tissues 28. PSA is considered as a reliable target for antibody-based approaches based on the correlation of PSA with tumor volume and tissue specificity and its suitable epitope distribution 20.
Conclusion :
In conclusion, this study reports production of five anti-PSA mAbs which may be useful for further development of diagnostic strategies for PSA analysis in biological materials.
Acknowledgement :
This study was supported by a grant (no.2025) from Scientific Research Council of Iran. The authors would like to thank Mrs. M. Babaee for technical assistance.
Conflict of Interest :
The authors have no conflicts of interest to declare.

Figure 1. SDS-PAGE analysis of PSA purified from seminal fluid by affinity chromatography. The purified PSA is shown as a ~33 kDa band.
|
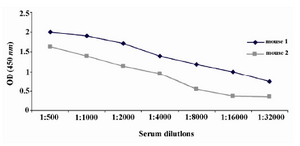
Figure 2. Titration of anti-PSA antibody in immunized mice sera by indirect ELISA. The results indicated that both mice were immunized with PSA. However, mouse 1 had higher titer of PSA-specific antibody and was selected for fusion.
|

Figure 3. Detection of the reactivity of produced anti-PSA mAbs with purified PSA; A) and human semen; B) by ELISA. Anti-PSA mAb produced by clone 2C8-E9 showed the highest reactivity with both purified PSA and seminal fluid.
|
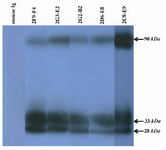
Figure 4. Detection of PSA in human semen using anti-PSA mAbs by western blot analysis. All produced anti-PSA mAbs could recognize a ~33 kDa band related to free PSA in the seminal fluids. Two other bands, 90 kDa and 28 kDa, were also found in WB. That can represent PSA-PCI complex and endoproteolytic cleavage product of PSA, respectively.
|
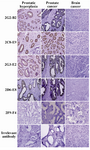
Figure 5. Assessment of anti-PSA mAb immunoreactivities in IHC. Sections of prostate cancer (PCa), benign prostatic hyperplasia (BPH) and brain cancer tissues were prepared and stained with anti-PSA mAbs (original magnification, ×20).
|
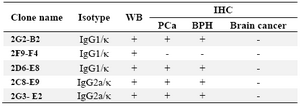
Table 1. Characterization and the reactivity of mAbs against PSA
WB: Western Blot; PCa: Prostate Cancer; BPH: Benign Prostatic Hyperplasia
|
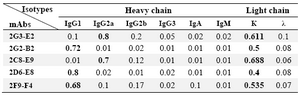
Table 2. Isotype determination of produced anti-PSA mAbs using a home-made ELISA
Values representing the highest OD obtained in ELISA are highlighted
|
|