Effects of Treatment with Platinum Azidothymidine and Azidothymidine on Telomerase Activity and Bcl-2 Concentration in Hepatocellular Carcinoma- Induced Rats
-
Sabokrouh, Abdolreza
-
Department of Clinical Biochemistry, School of Medicine, Hamadan University of Medical Sciences, Hamedan, Iran
-
 Goodarzi, Mohammad Taghi
Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 811 8380462; Email: mt.goodarzi@umsha.ac.ir
Goodarzi, Mohammad Taghi
Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamadan, Iran, Tel: +98 811 8380462; Email: mt.goodarzi@umsha.ac.ir
-
Research Center for Molecular Medicine, Hamadan University of Medical Sciences, Hamedan, Iran
-
 Vaisi-Raygani, Asad
Molecular Diagnostic Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran, Email:asadvaisiraygani@kums.ac.ir
Vaisi-Raygani, Asad
Molecular Diagnostic Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran, Email:asadvaisiraygani@kums.ac.ir
-
Molecular Diagnostic Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
-
Khatami, Shohreh
-
Department of Clinical Biochemistry, Pasteur Institute of Iran, Tehran, Iran
-
Taghizadeh-Jahed, Masoud
-
Department of Tissue Engineering, Reproductive Biotechnology Research Center, Avicenna Research Institute, ACECR, Tehran, Iran
Abstract: Background: Telomerase activity increases in cancer cells. Bcl-2 is an antiapoptotic factor that its concentration grows in many cancer cells including hepatocellular carcinoma cells. In this study, an attempt was made to investigate the effects of a new synthetic compound, platinum azidothymidine (Pt-AZT) on treatment of rats with Hepatocellular Carcinoma (HCC) and to compare its effects with azidothymidine (AZT) in alteration of telomerase activity and Bcl-2 concentration in HCC. Methods: Healthy adult male Wistar rats (n=100) were randomly divided into 4 groups (A, B, C, and D). Group A contained 25 healthy rats and was considered as the control group. Liver preneoplastic lesions were induced in remaining animals (n=75) using Solt-Farber resistant hepatocyte protocol. These animals were randomly allocated in groups B, C and D. Group B was negative control (untreated), groups C and D were treated by intraperitoneal injection (IP) of Pt-AZT (0.9 mg/kg/day) and AZT (0.3 mg/kg/day), respectively for 14 days. After the treatment period, telomerase activity and Bcl-2 concentration were determined in the rats’ liver. Results: No HCC was developed in group A, but tumors were present in all other groups. Telomerase activity and Bcl-2 concentration were significantly lower in group C compared to groups B (0.1590.06 vs. 0.5770.116 IU/L, p<0.001, respectively and 0.9310.388 vs. 3.940.74 ng/ml, p<0.001, respectively). Similar results were observed in comparison with group D (0.3310.06 vs. 0.5770.116 IU/L, p<0.001, respectively and 0.9310.388 vs. 2.940.594 ng/ml, respectively). There was a significant negative correlation between telomerase activity and Bcl-2 concentration only in untreated cancer group (p=0.034). Conclusion: In this study, higher anticancer activity of Pt-AZT in comparison to AZT was demonstrated. It effectively inhibits the growth of liver tumor in rats through extending apoptosis.
Introduction :
Hepatocellular Carcinoma (HCC) is a leading cause of death in Iran and many Asian countries and because of early metastasis and progression, its treatment is difficult. However, chemotherapy showed limited effect until now; therefore, other antitumor drugs are needed to be investigated.
Telomeres are tandem repetitive guanine rich sequences of TTAGGG at the end of chromosomes that are seen in non coding regions of DNA 1. Since DNA polymerase is not able to fully replicate 3′ end of DNA strand, the telomeres of somatic cells were progressively shortened by 50-200 bp with each mitotic division. Telomeres protect chromosomes from degradation by capping the ends of chromosomes. Thus progressive shortening of telomeres interferes with telomeric caps formation which ultimately leads to chromosomal instability and can increase tumor formation by an increase in rate of mutation of tumor suppressor genes and oncogenes 2-5. A ribonucleoprotein enzyme i.e. telomerase can build 3′end of chromosomes 6. Telomerase is a RNA-dependent DNA polymerase that consists of several subunits including template RNA (TERC) and a catalytic reverse transcriptase (TERT) that adds de novo repetitive sequences of telomeric DNA after each cell division, thus maintains function and length of telomere despite the telomere scrubbing that normally occurs during replication of chromosome 7,8. Cancer cells acquired indefinitely growth capacity and maintenance of telomeres by telomerase activity 9-14.
The telomerase activity is not detected in most somatic cells including normal hepatocytes but is detected in many cancer cells including HCC 15-17. For this reason, telomerase inhibition by some drugs is a novel approach to cancer therapy 18.
Bcl-2(B cell lymphoma protein-2) is an anti-apoptotic protein which is located in outer membrane of mitochondria and with another anti-apoptotic protein i.e. BclXL inhibits the release of cytochrome C from the mitochondria 19; as a result, Bcl-2 inhibits apoptosis in cancer cells and therefore has important role in development of cancer and resistance of cancer to some anticancer drugs. High expression of Bcl-2 is found in many cancer cells and mediates the resistance of cancers to chemotherapeutic drugs 20. Many anticancer drugs act in tumor via apoptosis; so Bcl-2 can interfere with them by blocking the cell death signals. Therefore, inhibition of Bcl-2 by new synthetic drugs will either restore the apoptotic process in tumor cell or sensitize them to drug treatment as it was seen in treatment of non Hodgkin`s lymphoma in human through inhibition of Bcl-2 by antisense oligonucleotide 21. In addition to antisense oligonucleotide 22, single chain antibodies can target Bcl-2 proteins family in tumor cells and increasingly sensitize tumor cells to chemotherapy 23.
Azidothymidine is an anticancer drug that not only competitively decreases telomerase activity in tumor cells via active anabolite azidothymidine triphosphate (AZTTP) and arrests the tumor cells by favoring apoptosis or inducing senescence 24,25, but also through decreasing Bcl-2 expression and concentration in tumor cells lowers the resistance of the cells to apoptosis and increases their sensitivity to this drug 26.
In the present study, an attempt was made to study telomerase activity and Bcl-2 concentration in HCC induced rats after treatment with platinum azidothymidine (Pt-AZT), a new synthetic platinum compound and to compare these effects with those of azidothymidine (AZT).
Materials and Methods :
Pathogen-free male Wistar rats (n=100) were purchased from Razi Institute (Karaj, Iran), and were maintained under standard conditions for acclimatization for two weeks. All animals had free access to industrialized food and water.
In this study, one hundred pathogen-free rats were divided randomly to 4 groups (each group contained 25 rats). Group A was considered as the control group (healthy rats). Liver preneoplastic lesions were induced in remaining animals (n=75) using Solt- Farber resistant hepatocyte protocol and the induction was approved by a pathological laboratory. These animals were randomly allocated in groups B, C and D. Group B was negative control (untreated).
Induction of preneoplastic lesions in rats: The rats in groups B, C and D received 200 mg/kg Body Weight (BW) of diethyl nitrosamine by IP injection for initiation the phase of hepatocarcinogenesis. After two weeks, they received 2-amino acetyl fleourene (2-AAF) 20 mg/kg BW six times as follows: before surgical procedure (2/3 partial hepatectomy), they received 4 doses on 4 consecutive days and the remaining 2 doses on days 2 and 4 thereafter 27. The solution of 2-AAF (10 mg/ml) was prepared by dissolving 300 mg of 2-AAF in 1 ml of dimethyl sulfoxide (DMSO); subsequently, it was briefly sonicated and 29 ml of 1% aqueous solution of highly viscous carboxymethylcellulose was added (CMC Product No: 419273, Sigma Aldrich). This solution was used for gavage administration, by the method of Van der Heijden et al 28 as shown in figure 1. Six weeks after IP injection of DEN, rats were subjected to biopsy of liver for pathological studies.
Histopathological studies: Paraffin-embedded blocks of samples were prepared in Pathology Division of Cancer Institute of Imam-Khomeini Medical Center from biopsy of thin slices of rat’s liver and then were stained using Hematoxylin and Eosin (H&E) procedure. After staining, the slides were reviewed for preneoplastic lesions by a pathologist in a blind manner. Each slide was reviewed for 3 min under a light microscope. Cytoplasm and nucleus of transformed cells were examined; necrosis and apoptosis status in nucleus (karyolysis, pyknosis, karyorrhexis) were recorded.
The drugs treatments and biochemical studies were started after confirming preneoplastic lesions in the animals.
Drugs treatment: After confirmation of preneoplastic lesions on rat’s liver, groups C and D were treated by IP of 0.9 mg/kg/day of Pt-AZT and 0.3 mg/kg/day of AZT respectively for 14 days. These drugs concentrations were selected according to Jeng et al’s 26 report and our preliminary studies. At the end of the protocol, all rats were gradually sacrificed and biochemical experiments were carried out as follows.
Telomerase activity assay: Telomerase activity was measured using commercially available kit according to the kit instruction (Telomerase activity kit, Roche Company Ltd. Germany, Cat. No. 11 854 666 910) using TRAP (Telomeric repeat amplification protocol).
Cell homogenate was prepared from each frozen tissue specimen. After centrifugation (1400 g, 4°C), the supernatant was collected for further analysis. Following measuring total proteins of prepared samples (Bradford method), the protein content of each sample was adjusted to 50 µg. PCR amplification was performed in these specimens using a thermocycler according to the manufacturer’s protocol. The principle of the assay contained highly specific amplification of telomerase-mediated elongation products combined with non-radioactive detection following an ELISA protocol. In the first step, telomerase added telomeric repeats (TTAGGG) to the 3` end of biotin labeled synthetic P1-TS and P2, generating PCR products with the telomerase-specific 6 nucleotide increments. In the next step of procedure, an aliquot of the PCR product was denatured and hybridized to a digoxigenin labeled telomeric repeat-specific detection probe. The resulting product was immobilized via the biotin labeled primer to a streptavidin-coated microplate. The immobilized PCR product was then detected with an antibody against digoxigenin (anti-DIG-POD) that was conjugated to peroxidase. Finally, the probe was visualized by virtue of peroxidase metabolizing TMB to form a colored reaction product. The absorbency of samples were read at 450 nm using an ELISA plate reader. The sensitivity of telomerase activity kit was less than 0.001 IU/L.
Measurement of Bcl-2 concentration: Bcl-2 concentration was measured using commercially available kit according to the kit instruction [Bcl-2 kit (biorbyt) Life science (USCNK) Company Inc UK Cat. No Orb52840]. The minimum detectable dose of rat Bcl-2 was typically less than 0.057 ng/mL.
Briefly, for this assay, thin slices of frozen tissue specimens were rinsed in ice cold of PBS buffer (0.02 ml/L, pH=7.0-7.2) to remove excess blood; subsequently, the homogenized tissue was prepared on ice using a motorized pestle until uniform consistency. The prepared supernatant was used for assay according to the kit instruction. The kit protocol was a sandwich enzyme immunoassay for quantitative measurement of Bcl2 in rat serum. After adding TMB substrate and stopping the reaction, absorbance was measured at 405 nm in an ELISA plate reader.
Statistical analysis: The SPSS statistical software package version 16 was used for the statistical analyses. A p<0.05 was considered significant. The nonparametric Mann-Whitney U-test was used to compare Bcl-2 concentration and telomerase activity between studied groups. The correlation between telomerase activity and Bcl-2 concentration in the studied groups was calculated using linear regression.
Results :
There was not any tumor in liver of healthy rats (group A) at any point of the study; while neoplastic lesions were gradually developed in all lobes of the rats’ liver in groups B, C and D following the neoplasm induction by Solt-Farber resistant hepatocyte model after 8 weeks. In microscopic study of untreated rats’ liver, there were several enlarged nucleuses that showed intensive synthetic phase of cell cycle in these hepatocytes indicating presence of malignant cells (Figures 2A and B).
In treated rats with platinum azidothymidine, there were many regions with karyolysis and pyknosis that were signs of apoptosis and also some regions showed necrosis. Furthermore, in some slides hemorrhage was observed that was not seen in group B (Figures 2C and D). In treated rats with azidothymidine, there were several karyolysis and pyknosis regions but apoptosis extension was less comparing to the treated rats with Pt-AZT (Figures 2E and F).
At the end of preneoplastic lesions induction protocol, there were a few small size lesions and with passing time there was an increase in both size and number of lesions. After drug treatments, size of some lesions was decreased and some small lesions disappeared; but the efficacy of Pt-AZT was higher as compared to those of AZT. There was no abnormal finding in hepatocyte of normal rat after treatment with AZT or Pt-AZT.
The Bcl-2 concentration and telomerase activity in the studied groups are shown in table 1. The lowest telomerase activity was found in group C (Pt-AZT treated rats). Comparing telomerase activity between groups C and B indicated a significant decrease in group C (p<0.001); also Bcl-2 concentration was significantly lower in group C as compared with group B (p<0.001). Similar results were observed comparing these factors between groups D and B indicating significant decrease in both factors in group D (p<0.001). Also comparison between C and D groups showed lower telomerase activity (p<0.001) and Bcl-2 concentration (p<0.001) in group C (Table 1). These results indicated lower telomerase activity and Bcl-2 concentration in Pt-AZT treated group comparing to AZT treated group. Therefore, Pt-AZT was a more potent inhibitor in hepatocellular carcinoma as compared with AZT.
The difference in telomerase activity between group A (healthy group) and group B was significant (0.018±0.011 vs. 0.5770.116 IU/L, p<0.001) and similar result was found for Bcl-2 concentration in these two groups (0.190.074 vs. 3.940.74 ng/ml, p<0.001). Our result in comparing telomerase activity between groups A and group D showed a significant difference (0.0180.011 vs. 0.3310.06 IU/L p<0.001), also similar results for Bcl-2 concentration was observed (0.190.074 vs. 2.940.594 p<0.001, Table 1).
Our results indicated a significant negative correlation between telomerase activity and Bcl-2 concentration in untreated rats (group B) (r=-0.43, p=0.034). However, there were no significant correlation between telomerase activity and Bcl-2 concentration in other studied groups (Table 2).
Discussion :
In this study, it was found that Pt-AZT was more effective than AZT in inhibition of HCC that was induced in rats using resistant hepatocyte Solt-Farber protocol. Our findings showed that telomerase activity and Bcl-2 concentration in Pt-AZT-treated rats was lower than those of AZT-treated rat (Table 1). Also similarly these factors were reduced in AZT-treated rats comparing to the untreated HCC group (Table 1).
A study reported that AZT blocks telomerase activity and effectively inhibits tumor growth and liver metastasis induced by the carcinogen diethyl-nitrosamine (DEN) in rats 26. Our finding indicted that telomerase activity increased after HCC development in untreated group but in the C and D groups that were treated with Pt-AZT and AZT respectively it was significantly decreased. These changes in telomerase activity can be a novel tumor biomarker to detect HCC either at primary or progressive stages. Some authors reported that HCC progression is due to oxidative stress by telomerase activity 29.
In our study, it was found that telomerase activity in Pt-AZT treated group was significantly lower as compared to AZT treated rats, which is due to presence of platinum that designates manifold anticancer effects of this compound.
There is a report showing that low concentration of platinum compound 2,3-dibromosuccinato [2-(methylaminomethyl)pyridine] platinum (II) (compound E) in treatment of a human hepatoma cell line (HepG2) had the strongest inhibition in telomerase activity and gradually reduced the telomere length, and finally caused apoptosis 30. Our findings confirmed some previously reported results, that platinum compounds and derivatives are more effective in inhibition of telomerase activity than the original compounds. Yamamoto et al prepared the combination of epirubicin-incorporating micelle NC-6300 and 1,2-diaminocyclohexane platinum (II) (oxaliplatin parent complex) in 44As3Luc cells and evaluated its antitumor activity in mice bearing 44As3Luc xenografts and showed the higher efficacy of it which was due to the presence of platinum and epirubicin-incorporating micelle NC-6300; as the later complex is a carrier to target cells 31. Shimada et al reported that increasing telomerase activity in HCC is accompanied by progression of malignancy 32.
Some reports show that in vitro treatment of tumor cells with azidothymidine decreased telomerase activity and expression and consequently decreased tumorigenicity and metastatic potential of tumor cells with a substantial increase in apoptotic nuclei and decrease in cell viability 33,34. Liu et al investigated the effect of AZT on human glioblastoma cells in vitro and reported that telomerase activity of these cells that were measured by TRAP assay was significantly reduced after treatment of these cells with 50-100 µmole AZT 35. They concluded that AZT inhibits telomerase and cyclin A that can inhibit passing of cells from G2 to M and S phases and suppresses proliferation of cancer cells 35. Our results indicate that telomerase may be an important target for therapy by controlling cell proliferation and growth. All these studies focused primarily on inhibiting telomerase activity which led to reduced cancer progression and in some cases suppression of it. Secondly, platinum derivatives of these compounds have an effective role in the repression of telomerase activity in comparison to original anticancer compounds. From these studies, it can be concluded that AZT effectively inhibits HCC in vivo. The relationship between Bcl-2 concentration and resistance to drug treatment remains controversial.
According to our results showing the lower Bcl-2 concentrations in Pt-AZT- treated rats compared to AZT-treated group, there was less resistance to the drug in the former group. Comparing the extend of apoptosis which was wider in Pt-AZT-treated group than AZT, it was shown that greater inhibition of Bcl-2 genes expression and concentration decline by Pt-AZT in comparison to AZT. Also according to our findings, it can be concluded that there is a relationship between Bcl-2 concentration and resistance to these drugs. Some studies indicated close inverse relationship between Bcl-2 concentrations and resistance to anticancer drugs 36,39. Beale et al showed a statistically significant inverse correlation between inhibition of cell line growth and Bcl-2 levels in human ovarian carcinoma cells treated with cisplatin; over-expression and therefore increased concentration of Bcl-2 in these cells led to resistance to cisplatin as compared to the control 40. However, some studies reported no significant correlation between Bcl-2 concentration and anticancer drugs resistance 41-44. In our study, it was concluded that the relationship depended on some factors such as nature of anticancer drug and the treated concentration.
As described in the results, our study confirmed a statistically significant negative correlation between Bcl-2 concentration and telomerase activity only in untreated HCC rats (group B, r=-0.43, p=0.034). But in other studied groups, there were no significant negative correlation between these two factors (Table 2); however, the relationship between telomerase activity and Bcl-2 expression and concentration was reported in some studies 45-47. Lida et al reported a possible relationship between telomerase activity and Bcl-2 expression in colorectal carcinoma 45. Elkak et al reported no relationship between telomerase activity and Bcl-2 expression in human breast cancer 46. Also Ohmura et al suggested that the Bcl-2 protein concentration was conversely correlated with telomerase activity (similar to our findings) and the biological role of Bcl-2 protein differs by degree of tumor aggressiveness in low grade tumor 47. Similar to the above mentioned study, induction of HCC in our studied animals was in primary level (preneoplastic lesions); therefore, the degree of tumor aggressiveness was in low grade and the inverse correlation between telomerase activity and Bcl-2 protein was statistically significant.
As it has been indicated in several studies, our results showed that telomerase activity increased after the development of HCC; it is possibly due to the effect of hTERT protein component of telomerase that regulates telomerase activity in chemically induced hepatocellular carcinoma 48. Also, increase in Bcl-2 concentration was reported after the development of HCC 49-53.
AZT inhibits synthesis of cancer genome by its active anabolite AZTTP (azidothymidine triphosphate) through chain termination mechanism which may inhibit telomerase activity competitively 54. Also, according to the pathological findings, treatment with AZT leads to a decrease in the expression of some genes such as telomerase and Bcl-2. It acts to arrest the cells with inducing senescence and apoptosis in tumor cells 55,56. Another report confirming these facts demonstrates that immortalization has a key role in cell concentration; high telomerase expression is present in 85-90.9% of tumor cells, and telomerase activity increases during the period of normal cells transition towards tumor cells 57. AZT, interrupting the reverse transcriptase of cells, blocks the cell cycle and inhibits replication of cells and cell growth 58,59. Also, AZT inhibits several kinds of enzymes in tumor cells among which some contribute to cell cycle regulation such as Mad1. Consequently, reduction of these cell cycle factors inhibits cell growth in S phase and the cell enters apoptosis phase which indicates that AZT is an effective anticancer drug 60-62.
Recently, Shah Abadi et al synthesized some antiviral drugs such as platinum complexes e.g. Pt-AZT 63. They studied interaction of Pt-AZT with DNA in vitro and showed that Pt-AZT breaks the backbone of DNA by intercalated mechanism and creation of non-covalent binding between adjacent bases in DNA; subsequently the cells undergo apoptosis 64,65. This effect along with synergistic effects of AZT 54-62 destroys cancer cells by Pt-AZT.
Platin-3-azido-2, 3-dideoxythymidine (Pt-AZT), is a synthetic compound that is made from cisplatin and AZT 63-65. Pt-AZT may inhibit telomerase and Bcl-2 in cancer cells and therefore induces apoptosis process. There is no published study showing in vivo effects of Pt-AZT upon HCC. To our knowledge, this is the first investigation to examine the in vivo effects of Pt-AZT in telomerase and Bcl-2 expression in HCC rats that can show their potential as a new drug for HCC treatment.
Conclusion :
Our animal model provided an environment for the study of inhibitory effects of Pt-AZT on the growth, progression and metastasis of HCC rat. Since this study was carried out in vivo, it can be considered as an advantage of the study which differs from most of the studies in this field. As a novel finding in this study, for the first time, it was demonstrated that Pt-AZT can reduce the Bcl-2 concentration and telomerase activity more effectively than AZT (the highest levels of Bcl-2 concentration and telomerase activities found in untreated HCC group). These data suggest that Pt-AZT effectively inhibits the growth of liver tumor in rats by extending apoptosis as compared to AZT. Furthermore, our study illustrated that Pt-AZT as a new anticancer drug in vivo can be more efficient than AZT. However, further studies are needed to shed light on the contribution of inhibitory effect of Pt-AZT on the growth of liver tumor in rats by extending apoptosis.
Acknowledgement :
This study is financially supported by Hamadan University of Medical Sciences, Hamadan, Iran. This is a part of A. Sabokrouh PhD thesis.
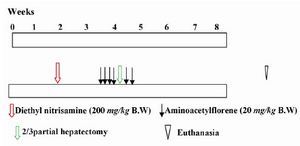
Figure 1. The levels of induction of Preneoplastic Lesion (PNL) in rats’ liver
|
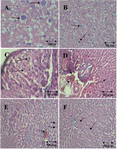
Figure 2. Cytological changes during preneoplastic lesion induction and after treatment with Pt-AZT and AT in rats’ liver (H&E staining method). A and B) rat liver with malignant cells. The arrows show the enlarged nucleuses of preneoplastic cells (original magnification 400× in A and 100× in B); C and D) rat liver after treatment with Pt- AZT. The arrows show disrupted nucleus (karyolysis) that is the sign of apoptosis in slide D) arrows also show necrosis and hemorrhage in some parts (original magnification 100×); E and F) rat liver treated with AZT. Arrows show karyolysis and pyknosis, also necrosis and hemorrhages are seen in some parts (original magnification 100×)
|
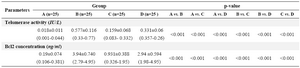
Table 1. The Bcl-2 concentration and telomerase activity in rat groups
A) Control group; B) Untreated cancer group; C) Cancer group treated with Pt-AZT; D) Cancer group treated with AZT; P-value: non-parametric test: Mann-Whitney U-test. Parenthesis: range
|

Table 2. Correlation between telomerase activity and Bcl-2 concentration in rat groups
|
|