Production of L-glutamic Acid with Corynebacterium glutamicum (NCIM 2168) and Pseudomonas reptilivora (NCIM 2598): A Study on Immobilization and Reusability
-
 Shyamkumar, Rajaram
Department of Biotechnology, Kamaraj College of Engineering and Technology, Virudhunagar, Tamil Nadu, India, Email: kingshyam2003@yahoo.co.in
Shyamkumar, Rajaram
Department of Biotechnology, Kamaraj College of Engineering and Technology, Virudhunagar, Tamil Nadu, India, Email: kingshyam2003@yahoo.co.in
-
Department of Biotechnology, Kamaraj College of Engineering and Technology, Virudhunagar, Tamil Nadu, India
-
Ponmurugan, Karuppiah
-
Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Kingdom of Saudi Arabia
-
Baskar, Rajoo
-
Department of Chemical Engineering, Kongu Engineering College, Perunduari, Erode, Tamil Nadu, India
Abstract: Background: L-glutamic acid is one of the major amino acids that is present in a wide variety of foods. It is mainly used as a food additive and flavor enhancer in the form of sodium salt. Corynebacterium glutamicum (C. glutamicum) is one of the major organisms widely used for glutamic acid production.
Methods: The study was dealing with immobilization of C. glutamicum and mixed culture of C. glutamicum and Pseudomonas reptilivora (P. reptilivora) for L-glutamic acid production using submerged fermentation. 2, 3 and 5% sodium alginate concentrations were used for production and reusability of immobilized cells for 5 more trials.
Results: The results revealed that 2% sodium alginate concentration produced the highest yield (13.026±0.247 g/l by C. glutamicum and 16.026±0.475 g/l by mixed immobilized culture). Moreover, reusability of immobilized cells was evaluated in 2% concentration with 5 more trials. However, when the number of cycles increased, the production of L-glutamic acid decreased.
Conclusion: Production of glutamic acid using optimized medium minimizes the time needed for designing the medium composition. It also minimizes external contamination. Glutamic acid production gradually decreased due to multiple uses of beads and consequently it reduces the shelf life.
Introduction :
L-amino acids are major biological components commercially used as additives in food, feed supplements, infusion compounds, therapeutic agents and precursors for peptides synthesis or agriculture based chemicals.The amino acids are the second most important category, after antibiotics, with fermentation products exhibiting the highest growth rates 1. L-glutamic acid was the first amino acid produced commercially. The substance was discovered and identified in the year 1866 by the German chemist Karl Heinrich Leopold Ritthausen. L-glutamic acid was mainly produced by microbial fermentations and the chemical mode of synthesis is not widely preferred due to the formation of racemic mixture 2.
In biotechnological processes, Corynebacterium species are used for economic production of glutamic acid by submerged fermentation 3. L-glutamic acid is produced per year using coryneform bacteria. A number of fermentation techniques have been used for the production of glutamic acid 4-6. Glucose is one of the major carbon sources for production of glutamic acid. Glutamic acid was produced with various kinds of raw materials using submerged fermentation of palm waste hydrolysate 7, cassava starch 8, sugar cane bagasse 6, date waste 9.
Immobilization of microbial cells in biological processes can occur either as a natural phenomenon or through artificial process. The method used for immobilization of cells was adsorption, cross linking, covalent bonding and encapsulation. These are all common methods employed for enzymes and microbial cells and usage of the methods depends on the cultures and conditions 10. Artificial immobilization of cells results in restricted growth and facilitates the production process. In biotechnology, it has been recognized that immobilization and co-immobilization of cells/ enzymes facilitates the feasibility of two or multi-step conversions into a single-step conversion. Binding of the deficient enzyme from an external source to free or immobilized microorganisms or immobilization of mixed culture capable of carrying out two or multistep conversions into a single-step conversion, leads to co-immobilized cells. The co-immobilized cells can open up new possibilities of synergistic action and result in more yield/conversion, which cannot be obtained to the same extent by separately immobilized cells 11,12. Hence, the present report focused on immobilization of whole cells of C. glutamicum and mixed culture of C. glutamicum and P. reptilivora for the production of glutamic acid with an optimized medium and reusability of immobilized cells for the production of glutamic acid.
Materials and Methods :
Media and chemicals: All media components of high purity were obtained from HiMedia Laboratories private limited, Mumbai, India. The remaining of all ingredients used was of analytical grade and the ingredients were purchased from Merck Limited, SD Fine chemicals limited, Mumbai, India. All media and chemicals were used without any pretreatment.
Microorganisms and inoculum: Stock culture of C. glutamicum (NCIM 2168) and P. reptilivora (NCIM 2598) was obtained from National Collection of Industrial Microorganisms (NCIM), Pune, India. Inoculum was prepared by transferring cells from agar slant into 250 ml flask containing 100 ml of the culture medium. Half (0.5) ml of each culture was taken and inoculated in the production medium and also used for immobilization studies.
Agar slant and culture medium: The constitution of the medium for preparing agar slant was kept at pH=7.0 and incubated at 30 C for at least three days. The slants were preserved at 4C and subcultured twice in a month.
Glutamic acid production and optimization: The medium composition for the production of glutamic acid was as the following: (g/l) Glucose-50.0, Urea-8.0, Biotin-0.002, K2HPO4-1.0, MgSO4.7H2O-2.5, MnSO4. 7H2O-0.1, CaCO3-1.6. The medium pH was adjusted to 7.0 with 1N sodium hydroxide or 1N hydrochloric acid. The fermentation was carried out in 250 ml Erlenmeyer flask. The fermentation medium was inoculated with 1% (v/v) of the overnight culture (C. glutamicum and equal volume of C. glutamicum and P. reptilivora mixed culture). The production medium was kept in an orbital incubator shaker at 30C at 120 rpm for 48 hr. Then the cells and debris were removed by centrifugation at 10000 g at 4oC for 10 min. Supernatants were used as the crude glutamic acid source for estimation.
The Response Surface Methodology (RSM) is a useful tool to make the design for various factors used for optimization of medium components in order to have a higher yield of glutamic acid production. The optimum medium components for glutamic acid production were reported in our previous work 13. Briefly, the effect of glucose, urea, salt solution and inoculum size were experimentally demonstrated for the production of glutamic acid. Second order quadratic model has been developed through RSM and it was validated statistically.
Genetic Algorithm (GA) was adopted for the optimization of RSM. The optimal conditions were provided with the use of glucose 49.99 g/l, urea 10 g/l, salt solution 18.06% (v/v), and inoculum size 4.99% (v/v). The amount of glutamic acid produced experimentally (19.69 g/l) was consistent with the predicted value (19.61 g/l) by genetic algorithm, and the model was proven to be good and exhibited high effectiveness. Hence, these optimal medium components were used for glutamic acid production using whole cell immobilization studies.
Glutamic acid estimation: Thin layer chromatography was employed for detecting L-glutamic acid in the culture medium and solvent system consisted of n-butanol: acetic acid: water (2:1:1). The visualization of spots was performed by spraying with 0.02% ninhydrin solution and the quantitative estimation of L-glutamic acid in the suspension was done using colorimetric method 14.
Immobilization: C. glutamicum and P. reptilvora cultures were grown in nutrient broth medium and centrifuged then washed with 0.01 M citrate buffer (pH=7.0). Next, cell count was determined by plating the suspended culture with serial dilutions. Then the cell count was adjusted in the range of 108 cells/ml. 5% (v/v) cell suspension was used as the inoculums 15. The cell suspension was slowly added to ether sterilized sodium alginate (2, 3 and 5% w/v) and mixed thoroughly with sterile glass rod. The mixture was continuously extruded into a 1 L flask containing 200 ml of 0.1 M CaCl2 solution through 10 ml glass syringe with a 22 gauge needle. The resulting beads were cured in 0.1 M CaCl2 for 30 min. Then the beads were washed aseptically with sterile buffer solution (pH=7) and with sterile distilled water. The immobilized cells were transferred to RSM-GA optimized medium and incubated in shaker at 30ºC and at 120 rpm.
Results :
Glutamic acid production: The production medium was inoculated with C. glutamicum and mixed culture of
C. glutamicum and P. reptilivora with appropriate inoculum size. First, glutamic acid yield was calculated for 24 to 72 hr. The preliminary study results showed that the mixed culture of C. glutamicum and P. reptilivora produced higher yield than C. glutamicum alone. The production was monitored for three consecutive days and is depicted in table 1. The higher yield was 5.42 g/l with C. glutamicum and 7.96 g/l by mixed culture. The incubation time did not have much influence on the production after 48 hr; hence, 48 hr incubation time was preferred for further experiments. Subsequently, RSM was used to optimize the medium components for production of glutamic acid both with C. glutamicum alone and with mixed culture of C. glutamicum and P. reptilivora. The optimized medium (Glucose-50 g/l, Urea-10 g/l, salt solution- 19.24%) was used along with standard concentration of biotin. Moreover, the optimized medium was chosen for immobilization studies.
Immobilization: Production of glutamic acid with immobilized C. glutamicum and immobilized mixed culture (C. glutamicum, P. reptilivora) was carried out and the effect of sodium alginate concentration was studied.
Effect of sodium alginate concentration and its reusability: Sodium alginate gel beads are easy to produce on a large scale without any sophisticated equipment. Different concentrations (2, 3 and 5% w/v) of sodium alginate immobilized cell beads were used for production of glutamic acid using the above mentioned optimized medium. Among the three concentrations of beads used, 2% alginate concentration beads produced the highest yield. Maximum glutamic acid yield was obtained at 2% sodium alginate concentration which was 13.026±0.247 g/l by C. glutamicum and 16.026±0.475 g/l by immobilized mixed culture (Table 2). Thus, the cultures immobilized with 2% sodium alginate concentration were taken for reusability studies. The immobilized beads were found to be stable up to 5 cycles. When the number of cycles increased, the production of L-glutamic acid decreased (Figure 1). The beads were disintegrated after the 5th cycle.
Discussion :
The preliminary reports of the present investigation revealed that the basic medium used for production of L-glutamic acid was lower than the optimized medium used for production of L-glutamic acid. In immobilization studies, sodium alginate concentration had an influence on density of the beads; higher alginate concentration showed lower conversion efficiency which might be due to reduced pore size of the beads. The lower sodium alginate concentration affects the leakage of biomass from the beads which could be due to increased pore size of the beads. In other studies, it has been reported that natural isolates of C. glutamicum was used for glutamic acid production with free whole cells and with immobilization. Comparatively, whole cells produced more glutamic acid than immobilized cells. Moreover, regarding glutamic acid production among immobilized cells, agarose produced more glutamic acid as compared to alginate 16. Another report emphasized that fed-batch and continuous fermentation process adopted for L-glutamic acid production with the cells of C. glutamicum entrapped in carrageenan gel. Higher yield was produced in batch fermentation rather than continuous fermentation process and repeated uses of immobilized cells resulted in lower glutamic acid production. Production was enhanced when the medium was supplemented with penicillin 6.
Sodium alginate concentration is also one of the factors influencing the productivity of immobilized cells. The reduction in productivity may be due to the increase in porosity which makes the leakage. Earlier investigations demonstrated that 3% alginate concentration enhances the productivity in co-immobilized culture of Brevibacterium roseum and E. coli among different con-centrations of alginate 17,18. The production of glutamic acid influenced immobilized cells due to ionic strength and stability in storage of beads 19,20. There are some more studies focused on pH, temperature, agitation and other physical parameters used in glutamic acid production with immobilization 21-25. This investigation analyzed reusability of immobilized cells for storage and usage in fermentation process. Furthermore, intensive studies are required for evaluating the methods in increasing glutamic acid production for immobilization in industrial fermentation.
Immobilization is highly sensitive to pH, temperature and other factors such as ionic potency in long incubation periods for nonspecific adsorption. The surface adherent cells can be removed due to these factors. Activation of surfaces with cross-linkers such as glutaraldehyde could lead to covalent attachment of the cells through surface amine groups. Loss of cell activity and viability in immobilization may be due to the formation of bonds with metal activated supports. Immobilization can be achieved by entrapping the cells within the matrix formed by gels made from alginates, carrageenans, and polyacrylamide materials.
Conclusion :
Immobilization of C. glutamicum and mixed culture of C. glutamicum and P. reptilivora was used for glutamic acid production. First, RSM was used to manipulate the medium components for enhanced production. Hence, it was easy to standardize the alginate concentration for immobilization. Two percent alginate concentration was fixed and reusability study was carried out to analyze the stability of beads for production of glutamic acid. This study demonstrated the procedures for economic production of glutamic acid. Cell entrapment in a polymer matrix such as sodium alginate has been widely used for commercial production of various products. The simple method adopted for entrapment of cells reduced the costs of production. In fact, it is very simple to collect and estimate the end product in the medium. It minimizes the external contamination and subsequently other effects in the fermentation process are minimized as well.
Acknowledgement :
The authors are grateful to the management and principal of Kamaraj College of Engineering and Technology, Virudhunagar, Tamil Nadu, for providing necessary facilities.
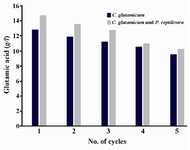
Figure 1. Reusability of immobilized cells with sodium alginate in glutamic acid production versus no. of cycles
|
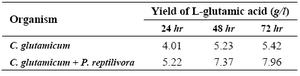
Table 1. Yield of glutamic acid at different incubation times
|
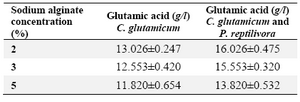
Table 2. The effect of sodium alginate on glutamic acid
production (immobilized cells)
|
|