An In vitro Study on Chick Somite Ability to Express Cerberus, Chordin, FGF8,Follistatin, and Noggin Transcripts
-
Karbalaie, Khadijeh
-
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran
-
Salehi, Hossein
-
Department of Anatomy, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran
-
Rabiee, Farzaneh
-
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran
-
 Ghaedi, Kamran
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran, Tel: +98 311 9515694; Email: kamranghaedi@royaninstitute.org
Ghaedi, Kamran
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran, Tel: +98 311 9515694; Email: kamranghaedi@royaninstitute.org
-
Cell and Molecular Biology Division, Department of Biology, School of Sciences, University of Isfahan, Isfahan, Iran
-
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran
-
 Nasr-Esfahani, Mohammad-Hossein
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran, Tel: +98 311 9515694; Email: mh_nasr@ royaninstitute.org
Nasr-Esfahani, Mohammad-Hossein
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran, Tel: +98 311 9515694; Email: mh_nasr@ royaninstitute.org
-
Department of Cellular Biotechnology at Cell Science Research Center, Royan Institute for Biotechnology, ACECR, Isfahan, Iran
Abstract: Background: In vitro simulation of developmental processes is an invaluable tool to shed light on the intrinsic mechanism of developmental biosystems such as central nervous system in mammals. Chick somites have been used to simulate the neural differentiation of human neural progenitor cells. In the present study, we aimed to indicate whether somites have the ability to express required neural differentiation factors at mRNA level.
Methods: Chick embryos were isolated from the yolk surface of the fertilized eggs and somites were subsequently isolated from embryos under a dissecting microscope. Total RNA of the somites was extracted and RT-PCR carried out with specific primers of cerberus, chordin, FGF8, follistatin and noggin.
Results: Data showed that five aforementioned factors were co-expressed after 7 days in vitro by somites.
Conclusion: We concluded that neural induction property of somites appeared by production of required neural differentiation factors including cerberus, chordin, FGF8, follistatin and noggin.
Introduction :
Somites are transient developmental structures and the derivatives of paraxial mesoderm which have an important role in organization of segmented pattern of vertebrate embryos 1. In vitro simulation of developmental mechanisms of vertebrates, especially mammals, could help us to find out the intrinsic molecular events involved in the development of organs and differentiation of various kinds of cells like CNS neurons. It is almost impossible to isolate developmental structures such as somite and notochord in human to study their functions in vitro. Even in laboratory mammals like mice, somites are too small to isolate and meticulous work should be done for separating them. Thus, the sole way is to separate such structures in chicken 2. In such situation, chick embryos are the best choice for somite isolation and in vitro simulation of developmental process of neurons. This simulation helps us to find out whether human embryonic cells respond to the molecular signals sent by somite, thereby exploring a new way for regeneration of damaged neural tissues. Previous studies 3 have shown that co-culture of somites derived from chick embryos of stages 9-12 of Hamburger and Hamilton 4 caused an increase in TUJ1/HOXB4 double positive group among human neural progenitors. Also it has been shown by Sagha et al 5 that somites maintain their neural induction ability in mouse embryonic stem cells. They have shown that somites thicken the adjacent part of neural tube, thereby affect the proliferation of neural tube precursors 6.
So far, there is no confirmation on expression of secretory factors by somite, whether they are able to co-express several neurogenic factors like noggin, chordin, follistatin, cerberus and FGF8 in vitro. The neurogenic activity of noggin 7, chordin 8, follistatin 9, cerberus 10 and FGF8 11 has already been established. Thus, this study investigated the co-expression of the mentioned factors by somites at mRNA level.
Materials and Methods :
Preparation of somite-containing alginate beads: Commercial chick eggs were provided through commercial sources and incubated in a humidified atmosphere at 38°C to yield the embryos at stages 9-12 as already described 12. Chick embryos were isolated from the yolk surface and transferred into Leibovitz’s (L15) medium (Invitrogen, USA). Then, embryos were dipped in dispase solution containing 1 mg dispase per 1 ml PBS (Invitrogen, USA) for 3-5 min for loosening chick embryo tissues. The enzyme was removed and embryos were washed with L15 medium supplemented with 5% Fetal Calf Serum (FCS; Invitrogen, USA) for 15 min. Subsequently, embryos were transferred into cold FCS free L15 medium. Somites were isolated from embryos under a dissecting microscope and transferred to medium. The presence of alginate beads facilitates the diffusing of secretory products of somites into the medium 13,14. These alginate beads helped somites to retain their integrity in vitro without somite cell migration in our media as well as to continue their intrinsic gene expression and protein secretion activity. Alginate beads were prepared according to previous reports 5.
RNA isolation and RT-PCR: Total RNA of the somites was extracted using an RNeasy kit (Qiagen, Germany) according to manufacturer's protocol. cDNA synthesis was done using a cDNA synthesis kit (TaKaRa, Japan) according to manufacturer’s instructions. Primer information is shown in table 1. PCR products were analyzed by gel electrophoresis on 1.5% agarose gel and stained with ethidium bromide (10 µg/ml), visualized and photographed by a UV transilluminator (Uvidoc, UK) (Figure 1).
Results :
RT-PCR on somite-derived cells revealed identifiable expression of cerberus, chordin, FGF8, follistatin and noggin after 7 days (Figure 1). The respective bands were not detected in no-template and no-primer tubes (data not shown).
Discussion :
This finding was strongly representing that these factors may be translated and exert a specific role in neural differentiation. Therefore, it seems that somites could retain their in vitro ability for expression of such factors at mRNA level.
In another study, it was reported that noggin 4 was expressed during the early development of the chick embryo even in gasrulation 15. This finding is consistent with our data that chick somite could express noggin. According to previous studies, it seems that neural induction ability of chick somite in human neural precursor cells 3 could be due to the expression and production of one of these factors: FGF8, chordin, cerberus, follistatin or noggin. However, due to presence of trace amounts of numerous factors in somite, detection of these factors requires a meticulous proteomic approach or secretome analysis of somites to find the real candidates responsible for induction of neural differentiation by somites.
In this study, we performed a conventional RT-PCR method as described to ensure that all aforementioned factors could be co-expressed appropriately by chick somites.
Conflict of Interest :
This study was approved by the ethical committee of Royan Institute. None of the authors has any conflicts of interest to disclose and all authors confirm the submission to this journal.
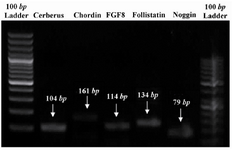
Figure 1. PCR products which were analyzed by gel electrophoresis evidencing that mentioned genes were expressed by chick somites in vitro
|
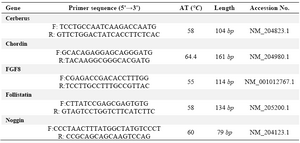
Table 1. Primers list in this study
F and R: are forward and reverse primers respectively. AT is annealing temperature which was set for the PCR for each primer pair. The length is related to the size of amplified product which is a partial segment of the coding sequences of the respected genes. Accession No refers to the registered No of each respected mRNA
|
|