Fine Structures of the Oocyte in Relation to Serum, Follicular Fluid Steroid Hormones and IGF-I in the Ovulatory-Sized Follicles in One-Humped Camel (Camelus dromedarius)
-
Kafi, Mojtaba
-
Department of Animal Reproduction, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
-
Mesbah, Seyed Fakhroddin
-
Department of Anatomical Sciences, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran
-
 Davoodian, Najmeh
Department of Animal Reproduction, School of Veterinary Medicine, Shiraz University, Shiraz, Iran, Email: najmeh179@gmail.com
Davoodian, Najmeh
Department of Animal Reproduction, School of Veterinary Medicine, Shiraz University, Shiraz, Iran, Email: najmeh179@gmail.com
-
Department of Animal Reproduction, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
-
Kadivar, Ali
-
Research Institute of Animal Embryo Technology, Shahrekord University, Shahrekord, Iran
Abstract: Background: The following study was carried out to determine the ultrastructural features of the oocyte of the ovulatory-sized follicles in relation to concentrations of steroids and IGF-I in the follicular fluid and serum in the dromedary camel.
Methods: Camel follicles with a clear and healthy appearance were categorized into three classes: follicles 10 to 13.9, 14-17.9 and 18-30 mm diameter. The Follicular Fluid (FF) and serum samples were assayed for estradiol-17β, progesterone and IGF-I. Recovered Cumulus-Oocyte Complexes (COCs) were prepared for transmission electron microscopy.
Results: The mean (±SD) FF concentrations of progesterone and IGF-I was significantly (p<0.05) higher in follicles 18 to 30 mm diameter compared to other groups of follicles. There was no difference in the mean (±SD) serum estradiol-17β, progesterone and IGF-I concentrations between camels with different ovulatory-sized follicles (p>0.05). Oocytes from follicles 18 to 30 mm diameter (group 3) showed more advanced signs of maturation including the disappearance of the nuclear envelope, increased number of microvilli in erect position, the increase in number and size of vesicles and more even distribution of the mitochondria throughout the ooplasm.
Conclusion: The final stages of oocyte maturation in dromedary camel is associated with increasing progesterone and IGF-I concentrations and constant high estradiol concentration in the follicular fluid which are paralleled with well-defined ultrastructural changes in oocytes.
Introduction :
The camel, a popular and economically important species performs well under arid and semi-arid climate conditions, still remains one of the most neglected species in the field of scientific research. The reproductive efficiency of camels under natural conditions is being reported to be low 1. Various attempts have been made to improve the reproductive efficiency and genetic potential of dromedary camel 2. Techniques such as in vitro production 3 and cloning 4 in the dromedary camel have been successfully carried out with a low success rate. Therefore, there is a serious need to perform more basic research regarding the biology of follicular development
and oocyte maturation in vivo. In addition, knowing the endocrinology and morphological aspects of the final stage of preovulatory follicle and oocyte maturation are the key elements for designing optimum in vitro conditions to develop oocyte and embryo culture systems.
Our study is the first to show the relationship between ultrastructure of the oocyte to hormone concentrations in the FF in Camelus dromedarius (C. dromedarius). The ultrastructural changes of the immature oocytes 5 and oocytes from different follicle sizes 6 were described in the dromedary caml. Kafi et al 7 also described the ultrastructural features of the oocytes during in vitro maturation in the dromedary camel. Therefore, the following study was carried out to determine the ultrastructural features of the oocyte of the ovulatory-sized follicles associated with the concentrations of steroids and IGF-I in the FF and serum in the dromedary camel.
Materials and Methods :
Ovaries were recovered from sexually mature non-pregnant camels (C. dromedarius) 6. During slaughtering, jugular blood samples were collected, centrifuged at 1400 for 30 min and stored in -20°C until analysis.
The ovulatory-sized follicles with a healthy and clear appearance were divided into three classes: 1) follicles 10-13.9 mm diameter, 2) follicles 14-17.9 mm diameter and 3) follicles 18-30 mm diameter. The follicles were aspirated and the cumulus-oocyte complexes were isolated under stereomicroscope. COCs were prepared for transmission electron microscopy as previously described 6. The FF samples were centrifuged at 3000×g for 15 min, decanted and stored in small aliquots at -20°C for hormone assay.
Estradiol 17-β, progesterone and IGF-I concentrations in the FF and serum were determined using a validated commercial radioimmunoassay kit (Immunotech kit, France). The differences in mean (±SD) hormone concentrations were statistically compared using a one-way ANOVA. Duncan's multiple range test was applied to find the significant differences in multiple mean comparisons using the SPSS software. Correlations between serum and FF hormone concentrations were assessed by correlation analysis. The level of significance was set at p<0.05.
Results :
The class size and the mean (±SD) follicular fluid concentrations of estradiol-17β, progesterone and IGF-I in different size follicles and the serum values of the hormones assayed are shown in table 1. The mean (±SD) follicular fluid concentrations of estradiol-17β, progesterone and IGF-I were significantly different (p<0.05) in follicles 10-13.9 mm diameter than follicles with 18-30 mm diameter. Further, the mean (±SD) FF concentrations of progesterone and IGF-I were significantly different (p<0.05) in follicles 14-17.9 mm diameter than that of follicles 18-30 mm diameter. There was no significant difference in the mean (±SD) follicular fluid concentrations of estradiol-17β between the follicles 14-17.9 and 18-30 mm diameter.
The Estrogen:Progesterone (E/P) ratio in the follicular fluid was significantly higher in follicles 10-13.9 mm diameter (59.07±14.12) than in the FF in the follicles 14-17.9 mm diameter (18.90±5.19) and 18-30 mm diameter (4.07±1.34) compared with 10 to 13.9 mm diameter. E/P ratio was not significantly different between follicles 14-17.9 mm diameter and follicles 18-30 mm diameter (Table 1).
The concentrations of estradiol-17β, progesterone and IGF-I in the serum were not correlated to those of the FF (r=0.23 for estradiol-17β, r=-0.03 for progesterone and r=0.33 for IGF-I; p>0.05), respectively. Also there were no differences between serum concentrations of estradiol-17β, progesterone and IGF-I of camels with different ovulatory-sized follicles (p>0.05).
Oocyte ultrastructure evaluation: Group 1- follicles 10-13.9 mm: The oocytes were surrounded by relatively expanded granulosa cells. Zona pellucida was homogenous containing numerous projections of cumulus cells. The nucleus was located eccentrically in half of the oocytes. The perivitelline space was small with limited numbers of microvilli. Large numbers of different size vesicles were distributed through the ooplasm. Clusters of mitochondria and Golgi complex were observed with peripheral distribution (Figure 1). Few numbers of lipid droplets were observed. Vesicles, mitochondria and lipid droplets were intermixed and located close to both vesicular and tubular endoplasmic reticulum in peripheral area.
Group 2- follicles 14-17.9 mm: Most ultrastructural features, in general, were similar to last group except that the PVS was slightly enlarged, the numbers of microvilli increased and the nucleus was peripherally located in all the oocytes (Figure 2).
Group 3- follicles 18-30 mm: All the oocytes showed no granulosa cells around. The number of microvilli was progressively increased (Figure 3). Cortical granules were also noted in the close proximity to the oolemma. The mitochondria were distributed through the ooplasm and the number and size of vesicles were observed to increase and distributed generally (Figure 4). The nuclear envelope was no longer apparent. The presence of the first polar body in the PVS was noted in one of the oocytes.
Discussion :
The results of the present study showed that as the diameter of the ovulatory-sized follicle increases, the mean concentrations of estradiol-17β, progesterone and IGF-I in the FF concomitantly increases in the female dromedary camel. Similarly, Rahman 8 reported that concentrations of estradiol-17β and progesterone in the follicular fluid increased with the growth of the follicles in the female dromedary camel. In the equine, Spicer et al 9 showed that estradiol-17β and IGF-I concentrations in the FF was greater in large ovulatory than in medium size follicles. In cattle, IGF-I stimulates estradiol synthesis which is important for final maturation of the preovulatory follicle and the oocyte 10. The concomitant increase of FF IGF-I and estradiol-17β, simultaneously with the increase in ovulatory-sized follicles in the present study suggests the presence of a similar relationship between IGF-I and estradiol-17β in the female dromedary camel as it occurs in the mare and cattle. Although a clear increasing pattern in serum concentrations of estradiol-17β and IGF-I was observed in camels with different ovulatory-sized follicles in the present study, this was not statistically significant due to the presence of the large standard deviations. The existence of a wide variation in blood steroid concentrations has been reported in the dromedary camel 11.
Our results showed that changes in the fine structure of the dromedary camel oocytes collected from preovulatory follicles are, in general, similar to that in cattle 12 and equine 13. Furthermore, the results of the present study revealed that oocytes from group 3 follicles showed more advanced signs of maturation as defined in the in vivo matured oocytes in cattle 12 and equine 13, and in vitro matured camel oocytes 7. The mean FF concentrations of estradiol and IGF-I were the highest in ovulatory-sized follicles (group 3) compared to the other follicle class size groups in the present study. Teissier 14 in the human, Grondahl 13 in the equine, Sirotkin 15 in the porcine and Zhao 16 in the rat reported that elevated estradiol in the follicular fluid indicate a more advanced stage of oocyte maturation.
We did not observe a massive formation of cortical granules in close proximity to the oolemma as the diameter of follicles increased. This was not similar to the well-defined dynamics of cortical granule formation in our previous observations during in vitro maturation in the dromedary camel oocyte 7. The reason for this different observation could be in vivo vs. in vitro environment, and or there may be more than one organelle involved in cortical granule formation 18. In the present study, in one oocyte from group 3, the first polar body had been fully extruded. This can be ascribed to the occurrence of an infertile mating and then surging in luteinizing hormone in days before slaughtering the animal. It has been shown that E/P ratio greater than 1 is a sign of healthy follicles and oocytes in cattle 19. Although E/P ratio decreased as the size of follicles increased in our study, however the ratio remained greater than 1. Considering the normal ultrastructure of the oocytes and E/P ratio in the FF greater than 1 observed in the present study, it can be inferred that E/P ratio greater than 1 is an indicative for the healthiness of follicles in the dromedary camel.
Conclusion :
In conclusion, the present study is the first to show that, the final stages of oocyte maturation in vivo is associated with increasing progesterone, estradiol-17β and IGF-I concentrations which are paralleled with ultrastructural changes leading to the final stages of oocyte maturation in the dromedary camel. Further, follicle diameter influences concentrations of progesterone, estradiol-17β and IGF-I in the FF in the dromedary camel.
Acknowledgement :
The authors would like to thank Professor M. Saeb for allowing us to perform hormone assay in his laboratory and Mr. A. Safavi for his technical assistance. The work was financially supported by Shiraz University.
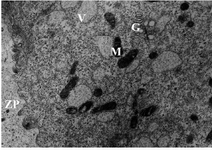
Figure 1. Electron micrograph showing details of an oocyte from group 1 follicles. Zona Pellucida (ZP), Mitochondria (M), Vesicles (V) and Golgi complex (G), ×11500
|
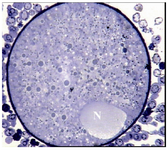
Figure 2. Light micrograph of an oocyte from group 2 which contains a peripherally located nucleus (N), ×720
|
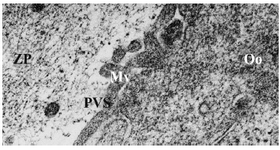
Figure 3. Electron micrograph of an oocyte from group 3. Microvilli (Mv), Perivitelline Space (PVS), Ooplasm (Oo), Zona Pellucida (ZP), ×21000
|
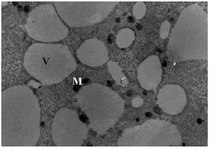
Figure 4. Electron micrograph of an oocyte from group 3, Mitochondria (M), Large vesicles (V), ×6600
|
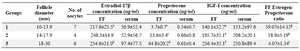
Table1. The mean±SD follicular fluid and serum concentrations of estradiol-17β, progesterone and IGF-I in ovulatory-sized follicles in the
dromedary camel
Values in the same column with different superscripts are significantly different
a,b) significant at p<0.07; c,d) significant at p<0.05; e,f) significant at p<0.01; g,h,i) significant at p<0.001
|
|